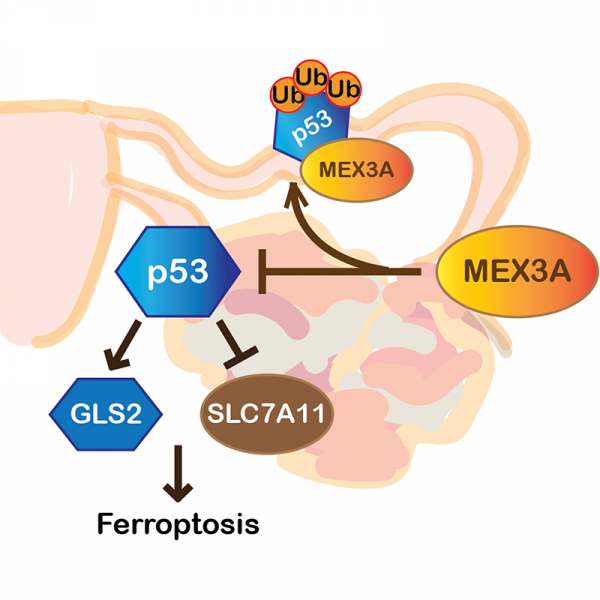
Epithelial ovarian cancer (OC) is a highly heterogeneous and malignant female cancer with an overall low survival rate. Mutations in p53 are prevalent in the major OC histotype, high-grade serous ovarian carcinoma (HGSOC), while p53 mutations are much less frequent in other OC subtypes, particularly in ovarian clear cell carcinoma (OCCC). Advanced stage OCCC with wildtype (WT) p53 has a worse prognosis and increased drug resistance, metastasis, and recurrence than HGSOC. The mechanisms responsible for driving the aggressiveness of WT p53-expressing OC remain poorly understood. Here, we found that upregulation of MEX3A, a dual-function protein containing a RING finger domain and an RNA binding domain, was critical for tumorigenesis in WT p53-expressing OC. MEX3A overexpression enhanced the growth and clonogenicity of OCCC cell lines. In contrast, depletion of MEX3A in OCCC cells, as well as ovarian teratocarcinoma cells, reduced cell survival and proliferative ability. MEX3A depletion also inhibited tumor growth and prolonged survival in orthotopic xenograft models. MEX3A depletion did not alter p53 mRNA level but did increase p53 protein stability. MEX3A-mediated p53 protein degradation was crucial to suppress ferroptosis and enhance tumorigenesis. Consistently, p53 knockdown reversed the effects of MEX3A depletion. Together, our observations identified MEX3A as an important oncogenic factor promoting tumorigenesis in OC cells expressing WT p53.
Language
週四, 10 十一月 2022 08:35