翁啟惠, 馬徹
Journal of the American Chemical Society
March 25, 2025
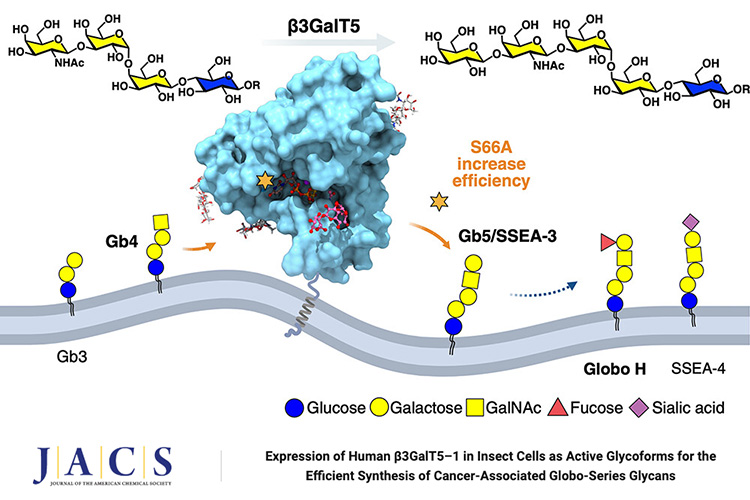
The globo-series glycosphingolipids (GSLs) are unique glycolipids exclusively expressed on the cell surface of various types of cancer and have been used as targets for the development of cancer vaccines and therapeutics. A practical enzymatic method has been developed for the synthesis of globo-series glycans, where the conversion of Gb4 to Gb5 (SSEA-3) glycan based on the microbial galactosyltransferase LgtD is relatively inefficient compared to other steps. To improve the efficiency, we explored the two human galactosyltransferase (β3GalT5) isozymes in cancer cells for this reaction and found that isozyme 1 (β3GalT5–1) is more active than isozyme 2 (β3GalT5–2). We then identified a common soluble domain of the two β3GalT5 isozymes as a candidate and evaluated the activity and substrate specificity of the glycosylated and nonglycosylated glycoforms. The glycoforms expressed in Sf9 cells were selected, and a site-specific alanine scan was performed to identify S66A β3GalT5 variant with 10-fold more efficiency than LgtD for the synthesis of globo-series glycans. The X-ray structure of β3GalT5–1 was determined for molecular modeling, and the result together with kinetic data were used to rationalize the improvement in catalysis.


