| Name | Eric Chi-Ching Cheung, Ph.D. |
|---|---|
| Incumbent | Assistant Professor |
| 所屬單位 |
Tumor-Host Interaction, Cancer Metabolism, Oxidative Stress, Tumor Microenvironment, Metastasis PhD, University of Ottawa, Ottawa ON, Canada 2008 BSc, University of British Columbia, Vancouver BC, Canada 2001 |
| NCLscan: |
|
| 研究方向/領域 |
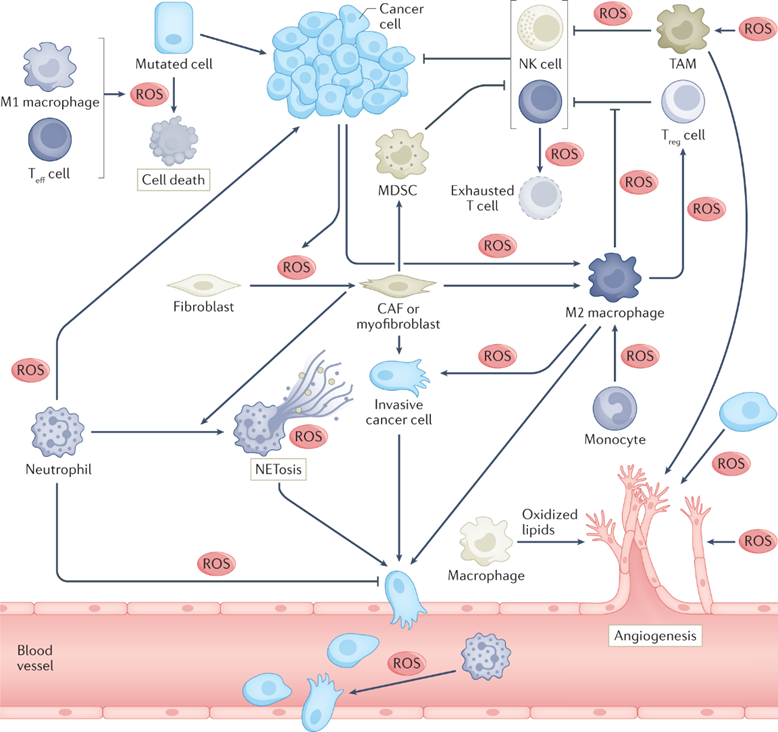
RESEARCH INTERESTSDecoding the Dialogue Between Cancer and the Host Cancer progression is not just a story about malignant cells; it's a story about a hostile takeover of the body. At the heart of this takeover is a fundamental rewiring of cellular metabolism. As cancer cells grow uncontrollably, they generate high levels of metabolic by-products called reactive oxygen species (ROS), leading to a state of oxidative stress. This stress can be a double-edged sword: while highly damaging, it can also trigger signaling pathways that cancer cells exploit to survive and spread. A critical question in cancer biology is how tumors manage this delicate balance. Our laboratory operates on the central premise that cancer cells cannot solve this problem alone. They engage in a constant, dynamic dialogue with the non-cancerous cells and organs of the host. We believe that by decoding this communication, we can find new ways to cut the lines of support for tumors to grow and metastasize. Our research moves beyond the cancer-cell-centric view to investigate how these critical tumor-host interactions—both locally within the tumor microenvironment and systemically with distant organs—dictate the course of cancer. To address these questions, we integrate sophisticated in vivo genetically-altered mouse models with advanced ex vivo organoid co-culture systems. These models are interrogated with powerful technologies, including single-cell RNA sequencing, CRISPR-based somatic genome editing, and metabolomics, allowing us to dissect these complex interactions with unprecedented resolution. Ultimately, our goal is to translate our findings into more refined and effective cancer therapies. By including the host side of the tumor-host interaction as additional targets, we aim to develop novel strategies that disrupt the pro-tumor support network, creating a new therapeutic window to combat cancer progression and resistance.
|
EDUCATION AND POSITIONS HELD
- 2025-present Assistant Professor, Genomics Research Center, Academia Sinica, Taipei, Taiwan
- 2022-2025 Principal Research Laboratory Scientist, The Francis Crick Institute, London, UK Group Leader: Karen Vousden
- 2018-2022 Senior Research Laboratory Scientist, The Francis Crick Institute, London, UK Group Leader: Karen Vousden
- 2014-2018 Associate Scientist, Cancer Research UK Beatson Institute, Glasgow, UK Group Leader: Karen Vousden
- 2009-2014 Post-doctoral Fellow, Cancer Research UK Beatson Institute, Glasgow, UK Group Leader: Karen Vousden
HONORS
- 2009-2012 Canadian Institutes of Health Research (CIHR) Post-doctoral Fellowship
- 2005-2008 Canadian Institutes of Health Research (CIHR) Doctoral Research Award
- 2008 The Governor General's Academic Gold Medal, Canada
- 2005, 2007 Brain Star Award, CIHR Institute of Neurosciences, Mental Health and Addiction
-
Cheung EC, Strathdee D, Stevenson D, Coomes J, Blyth K, Vousden KH. Regulation of ROS signaling by TIGAR induces cancer-modulating responses in the tumor microenvironment.
PNAS 2024 Dec 10;121(50): e2416076121 - Hennequart M, Pilley SE, Labuschagne CF, Coomes J, Mervant L, Driscoll PC, Legrave NM, Lee Y, Kreuzaler P, Macintyre B, Panina Y, Blagih J, Stevenson D, Strathdee D, Schneider-Luftman D, Grönroos E, Cheung EC, Yuneva M, Swanton C, Vousden KH. ALDH1L2 regulation of formate, formyl-methionine, and ROS controls cancer cell migration and metastasis.
Cell Report 2023 May 26;42(6):112562 - Zani F, Blagih J, Hennequart M, Jones N, Pilley S, Barrio PS, Legrave N, Cheung EC, Buck MD, Vousden KH. The dietary sweetener sucralose is a negative immunomodulator of T cell mediated responses.
Nature 2023; Mar 15;615(7953):705-711 -
Cheung EC, Vousden KH. The role of ROS in tumour development and progression.
Nat. Rev. Cancer 2022; 22(5):280-297 -
Cheung EC, DeNicola GM, Nixon C, Blyth K, Labuschagne CF, Tuveson DA, Vousden KH. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer.
Cancer Cell 2020; 37(2):168-182 - Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization.
Cell Metabolism 2019; 30(4):720-734 - Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, Blagih J, Vincent DF, Campbell KJ, Ceteci F, Sansom OJ, Blyth K, Vousden KH. Modulating the therapeutic response of tumours to dietary serine and glycine starvation.
Nature 2017; Apr 19;544(7650):372-376 -
Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom O, Vousden KH. Opposing effects of TIGAR and RAC1 derived ROS on Wnt driven proliferation in the mouse intestine.
Genes & Development 2016; Jan 1;30(1):52-63 -
Cheung EC, Athineos D, Lee P, Ridgway RA, Lambie W, Nixon C, Strathdee D, Blyth K, Sansom OJ, Vousden KH. TIGAR is required for intestinal regeneration and tumorigenesis.
Developmental Cell 2013; 25(5):463-77