| 姓名 | 張志禎博士 |
|---|---|
| 現任 | 助研究員 |
| 所屬單位 |
腫瘤-宿主交互作用, 癌症代謝, 活性氧化壓力, 腫瘤微環境, 轉移 PhD, University of Ottawa, Ottawa ON, Canada 2008 BSc, University of British Columbia, Vancouver BC, Canada 2001 |
| 名片 |
|
| 研究方向/領域 |
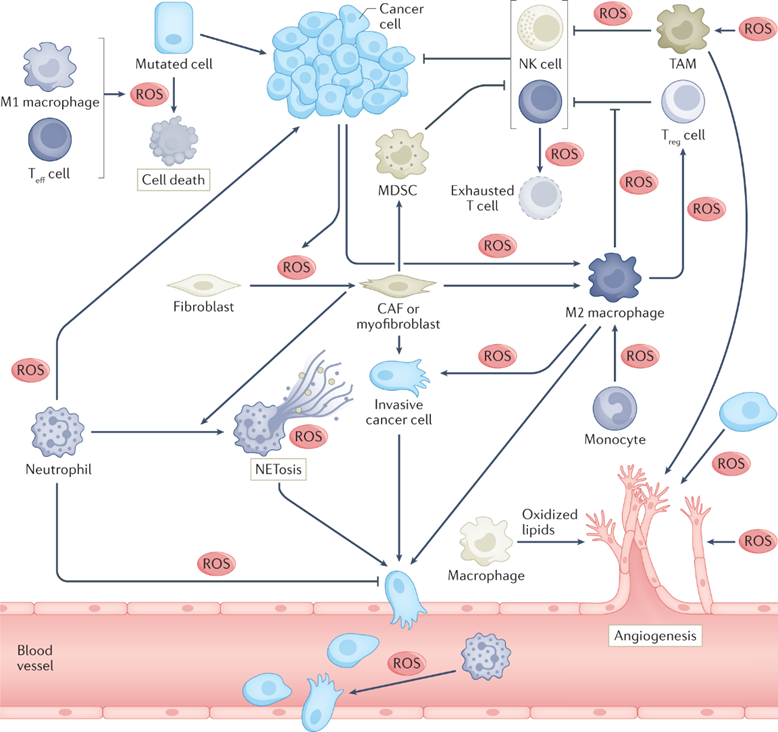
研究方向/領域解碼癌症與宿主之間的對話 癌症的進展不僅僅是關乎腫瘤細胞自身的變異,更是一場對身體各方面的惡性操控。這場奪取的核心機制在於對異常新陳代謝的適應性變化。當癌細胞不受控制地生長時,會產生大量活性氧為代謝副產物,從而進入氧化應激狀態。這種狀態是一把雙面刃:活性氧既能造成損傷而減慢癌細胞生長,也能啟動特定的訊息傳導讓癌細胞賴以存活和擴散。因此,癌症生物學的一個關鍵問題是,腫瘤如何巧妙地維持這種微妙的平衡。 我們實驗室的核心理念是:癌細胞無法獨自解決這些難題。它們必須與體內正常的非癌細胞及器官進行持續且動態的對話。透過解構這些聯繫機制,我們就能找到新的方法,切斷腫瘤所依賴的支援網絡。我們的研究超越了以腫瘤為中心的觀點,深入探討這些關鍵的腫瘤-宿主交互作用—無論是在局部的微環境中,還是在整體上與遠端器官的互動—如何決定癌症的演變。 我們的研究奠基於氧化應激對癌細胞的影響,高度取決於該腫瘤從何器官而來。以代謝調控因子TIGAR為例,我們發現調控活性氧的水平,可以在腸道中抑制腫瘤生長,但矛盾的是,在胰腺癌中卻會促進其轉移。這種顯著的差異,說明了周圍宿主環境對腫瘤的強大影響。 我們實驗室整合精密的基因改造小鼠模型與先進的類器官體外共培養系統,並利用單細胞RNA定序、CRISPR體細胞基因編輯以及代謝體學等強大技術,去深入剖析這些複雜的交互作用。 我們的目標是將研究成果轉化為更精準、更有效的癌症療法。透過靶向「腫瘤-宿主交互作用」中的宿主端,我們期望能瓦解腫瘤惡性操控宿主的方式,為對抗癌症的進展與抗藥性,開創一個全新的治療契機。
|
經歷
- 2025-present Assistant Professor, Genomics Research Center, Academia Sinica, Taipei, Taiwan
- 2022-2025 Principal Research Laboratory Scientist, The Francis Crick Institute, London, UK Group Leader: Karen Vousden
- 2018-2022 Senior Research Laboratory Scientist, The Francis Crick Institute, London, UK Group Leader: Karen Vousden
- 2014-2018 Associate Scientist, Cancer Research UK Beatson Institute, Glasgow, UK Group Leader: Karen Vousden
- 2009-2014 Post-doctoral Fellow, Cancer Research UK Beatson Institute, Glasgow, UK Group Leader: Karen Vousden
榮譽
- 2009-2012 Canadian Institutes of Health Research (CIHR) Post-doctoral Fellowship
- 2005-2008 Canadian Institutes of Health Research (CIHR) Doctoral Research Award
- 2008 The Governor General's Academic Gold Medal, Canada
- 2005, 2007 Brain Star Award, CIHR Institute of Neurosciences, Mental Health and Addiction
-
Cheung EC, Strathdee D, Stevenson D, Coomes J, Blyth K, Vousden KH. Regulation of ROS signaling by TIGAR induces cancer-modulating responses in the tumor microenvironment.
PNAS 2024 Dec 10;121(50): e2416076121 - Hennequart M, Pilley SE, Labuschagne CF, Coomes J, Mervant L, Driscoll PC, Legrave NM, Lee Y, Kreuzaler P, Macintyre B, Panina Y, Blagih J, Stevenson D, Strathdee D, Schneider-Luftman D, Grönroos E, Cheung EC, Yuneva M, Swanton C, Vousden KH. ALDH1L2 regulation of formate, formyl-methionine, and ROS controls cancer cell migration and metastasis.
Cell Report 2023 May 26;42(6):112562 - Zani F, Blagih J, Hennequart M, Jones N, Pilley S, Barrio PS, Legrave N, Cheung EC, Buck MD, Vousden KH. The dietary sweetener sucralose is a negative immunomodulator of T cell mediated responses.
Nature 2023; Mar 15;615(7953):705-711 -
Cheung EC, Vousden KH. The role of ROS in tumour development and progression.
Nat. Rev. Cancer 2022; 22(5):280-297 -
Cheung EC, DeNicola GM, Nixon C, Blyth K, Labuschagne CF, Tuveson DA, Vousden KH. Dynamic ROS Control by TIGAR Regulates the Initiation and Progression of Pancreatic Cancer.
Cancer Cell 2020; 37(2):168-182 - Labuschagne CF, Cheung EC, Blagih J, Domart MC, Vousden KH. Cell Clustering Promotes a Metabolic Switch that Supports Metastatic Colonization.
Cell Metabolism 2019; 30(4):720-734 - Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, Blagih J, Vincent DF, Campbell KJ, Ceteci F, Sansom OJ, Blyth K, Vousden KH. Modulating the therapeutic response of tumours to dietary serine and glycine starvation.
Nature 2017; Apr 19;544(7650):372-376 -
Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom O, Vousden KH. Opposing effects of TIGAR and RAC1 derived ROS on Wnt driven proliferation in the mouse intestine.
Genes & Development 2016; Jan 1;30(1):52-63 -
Cheung EC, Athineos D, Lee P, Ridgway RA, Lambie W, Nixon C, Strathdee D, Blyth K, Sansom OJ, Vousden KH. TIGAR is required for intestinal regeneration and tumorigenesis.
Developmental Cell 2013; 25(5):463-77