 Team members: PhD candidate Yu-Sheng Fang, assistant research fellow Dr. Yun-Ru Ruby Chen, research assistant Yu-Jen Chang(from left to right)For nearly 30 years, inclusions attributed from protein misfolding have been confirmed in the study of neurodegenerative diseases, be it Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease . For example, two proteins, Amyloid-beta (Aβ) and Tau, are identified as the causes of AD; only until 2006 has a protein named TDP-43 started to emerge as potentially a new disease causing target to pound on.
Team members: PhD candidate Yu-Sheng Fang, assistant research fellow Dr. Yun-Ru Ruby Chen, research assistant Yu-Jen Chang(from left to right)For nearly 30 years, inclusions attributed from protein misfolding have been confirmed in the study of neurodegenerative diseases, be it Alzheimer’s disease (AD), Parkinson’s disease, Huntington’s disease . For example, two proteins, Amyloid-beta (Aβ) and Tau, are identified as the causes of AD; only until 2006 has a protein named TDP-43 started to emerge as potentially a new disease causing target to pound on.
Current studies focus on a truncated form of TDP-43 protein, it has been associated with subtypes of ALS and FTLD. Although the two diseases have totally different symptoms, while ALS patients maintain sharp memories yet FTLD patients have dementia at earlier ages; studies have identified TDP-43 in both patients’ inclusions. FTLD is the acronym for Fronto-temporal Lobar Dementia, meaning the inclusions appear in the front and side parts of the brain, and it is a different disease from AD.
Dr. Yun-Ru (Ruby) Chen has studied protein misfolding and neurodegenerative diseases for years, in her latest research article published in Nature Communications, she has successfully demonstrated that as opposed to previous findings, the full-length TDP-43 protein is actually toxic and may be the cause of amyloid oligomers that tangles FTLD patients’ frontal lobe nerve ends. Her team has demonstrated the proof by first creating an antibody, and then verified by various means, including confirmation with human FTLD brain tissues.
The result of their study has suggested a turnaround direction toward current scientific researches, and her finding is the first of its kind. Previously, studies have identified TDP-43 segments in ALS and FTLD patients, the suggested scenarios being the truncated form of TDP-43 cause fiber like formation in the brain neural cells, and eventually lead to the death of the neural cells.
In Chen’s study, first of all, they generated full-length TDP-43 protein from human gene sequence in E. coli. They have spotted that the full-length TDP-43 has shown a tendency to curl into ball-like structures. Further, by mixing it with an antibody which has been used to identify amyloid oligomers which appear mostly in Alzheimer's disease patients, they have seen positive reactions. Thus, they decided to go with the instinct and think out of the box.
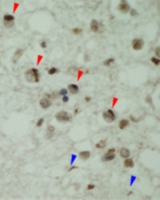 Immunohistochemical staining of TDP-43 in the FTLD-TDP patients by the TDP-O antibody shows inclusions (red arroweheads).The fiber-like substances found in Alzheimer's patients were considered less harmful to neuronal cells than amyloid oligomers. The group wants to see if TDP-43 has anything to do with this. By adding a merely 4% of the full-length TDP-43 to Aβ, amazingly, the fibers stopped forming, but only tiny ball-like amyloid oligomers were formed. Therefore, it appears TDP-43 has amyloid properties and may have played a role in the formation of amyloid oligomers in Alzheimer’s disease!
Immunohistochemical staining of TDP-43 in the FTLD-TDP patients by the TDP-O antibody shows inclusions (red arroweheads).The fiber-like substances found in Alzheimer's patients were considered less harmful to neuronal cells than amyloid oligomers. The group wants to see if TDP-43 has anything to do with this. By adding a merely 4% of the full-length TDP-43 to Aβ, amazingly, the fibers stopped forming, but only tiny ball-like amyloid oligomers were formed. Therefore, it appears TDP-43 has amyloid properties and may have played a role in the formation of amyloid oligomers in Alzheimer’s disease!
To further verify the existence of full-length TDP-43 oligomer, the team decided to create a specific antibody to do so. They injected full-length TDP-43 to lab rabbits and obtained polyclonal antibody. They named this antibody TDP-O.
As a result, various tests showed TDP-O has a high specificity. Collaborator Prof. Kuen-Jer Tsai from National Cheng Kung University used TDP-O and confirmed TDP-43 oligomers exist in transgenic mice brains, and the match increases with age of the mice.
To verify if TDP-43 oligomers are present in FTLD-TDP patients, by collaborating with Prof. Lee-Way Jin from the Alzheimer’s Disease Center of U.C. Davis Medical Center, they used TDP-O antibody and validated positive signals. These validations were all done at collaborator sites.
To double confirm, after obtaining the diseased human hippocampus tissue from Prof. Jin of U.C. Davis, they used TDP-O to immunoprecipitate the proteins from the diseased hippocampal tissues in their own lab, they were so excited to see with their own eyes, TDP-O has revealed abundant of TDP-43 oligomers in these human FTLD tissues.
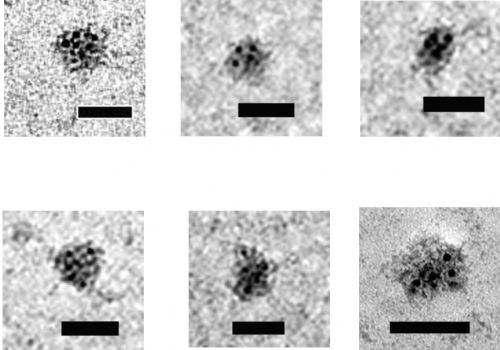 |
| The hippocampus tissue of a FTLD-TDP patient immunoprecipated with TPD-O antibody revealed spherical TDP-43 oligomers clearly under electron microscope. |
To Dr. Ruby Chen, this study has really come a long way! With accumulated knowledge in neurodegenerative diseases and collaboration with multiple parties, the scope of her lab has evolved from pure protein research to a step closer to find solutions to cure the patients. The study was done with collaboration with National Cheng Kung University, National Yang Ming University, Institute of Biomedical Sciences and Institute of Molecular Biology of Academia Sinica, and University of California Davis Medical Center. The published journal paper can be read on line at:http://www.nature.com/ncomms/2014/140912/ncomms5824/full/ncomms5824.html.
 |
| The Ruby Chen's Lab takes the Ice Bucket Challenge. |