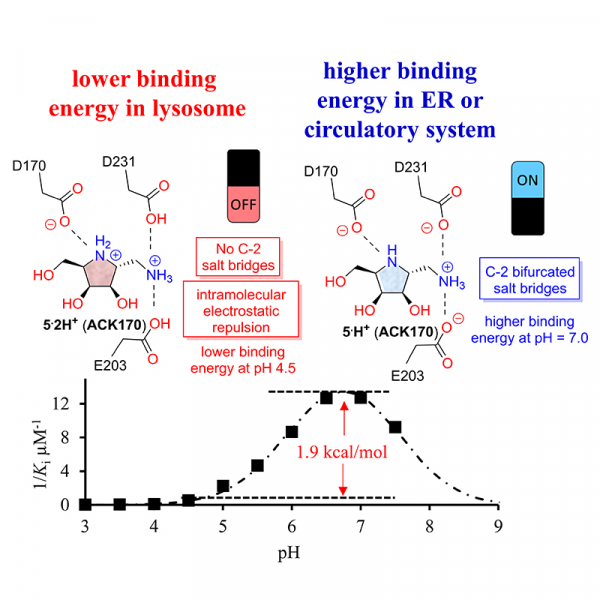
The use of pharmacological chaperones (PCs) to stabilize specific enzymes and impart a therapeutic benefit is an emerging strategy in drug discovery. However, designing molecules that can bind optimally to their targets at physiological pH remains a major challenge. Our previous study found that dibasic polyhydroxylated pyrrolidine 5 exhibited superior pH-selective inhibitory activity and chaperoning activity for human α-galactosidase A (α-Gal A) compared with its monobasic parent molecule, 4. To further investigate the role of different C-2 moieties on the pH-selectivity and protecting effects of these compounds, we designed and synthesized a library of monobasic and dibasic iminosugars, screened them for α-Gal A-stabilizing activity using thermal shift and heat-induced denaturation assays, and characterized the mechanistic basis for this stabilization using X-ray crystallography and binding assays. We noted that the dibasic iminosugars 5 and 20 protect α-Gal A from denaturation and inactivation at lower concentrations than monobasic or other N-substituted derivatives; a finding attributed to the nitrogen on the C-2 methylene of 5 and 20, which forms the bifurcated salt bridges (BSBs) with two carboxyl residues, E203 and D231. Additionally, the formation of BSBs at pH 7.0 and the electrostatic repulsion between the vicinal ammonium cations of dibasic iminosugars at pH 4.5 are responsible for their pH-selective binding to α-Gal A. Moreover, compounds 5 and 20 demonstrated promising results in improving enzyme replacement therapy and exhibited significant chaperoning effects in Fabry cells. These findings suggest amino-iminosugars 5 and 20 as useful models to demonstrate how an additional exocyclic amino group can improve their pH-selectivity and protecting effects, providing new insights for the design of pH-selective PCs.