研究員
研究員
中心副主任
EDUCATION AND POSITIONS HELD:
- B.S., Department of Agricultural Chemistry, National Taiwan University, Taiwan, 1992-1996
- Ph.D., Department of Molecular and Structural Biochemistry, North Carolina State University, USA, 1998-2003
- Research Assistant, Institute of Botany, Academia Sinica, 1996-1998
- Teaching Assistant, Dept. of Molecular and Structural Biochemistry, North Carolina State University, USA, 1998-1999
- Postdoctoral Fellow, Dept. of Molecular Biology & Biochemistry, University of California, Irvine, USA, 2004-2006
- Postdoctoral Fellow, Genomics Research Center, Academia Sinica, Taiwan, 2006-2007
- Assistant Professor, Genomics Research Center, Academia Sinica, Taiwan, 2007-2014
- Council Member of Asia Pacific Protein Association (APPA), 2014-2017
- The World Academy of Sciences (TWAS) Young Affiliate, 2015-2019
- Associate Professor, The Genomics Research Center, Academia Sinica, 2014-2021
- Adjunct Associate Professor, Dept. of Biochemical Science & Technology, National Taiwan University, Taiwan, 2015-present
- Professor, The Genomics Research Center, Academia Sinica, 2021-present
- Adjunct Professor, Dept. of Biochemical Science & Technology, National Taiwan University, Taiwan, 2021-present
- President-elect, Asia Pacific Protein Association (APPA), 2022-2025
- Adjunct Professor/ Adjunct Research Fellow, Biomedical Translation Research Center, Academia Sinica, Taiwan, 2022-present
- Deputy Director, Genomics Research Center, Academia Sinica, 2023-present
HONORS:
- Chung Hwa Rotary Education Foundation Outstanding Award, 2022-2023
2023 中華扶輪教育基金會2022-23年度「傑出/特殊人才」獎 - The 19th National Innovation Award. “A novel conformation-dependent monoclonal antibody against ALS and related neurodegenerative diseases”, 2022
2022 第19屆國家新創獎,「對抗漸凍人及相關神經退化疾病的結構專一型單株抗體」。 - The 19th Y. Z. Hsu Scientific Paper Award, Far Eastern Y. Z. Hsu Science and Technology Memorial Foundation, 2021
2021第十九屆有庠科技論文獎 - National China Youth Corps Youth Medal, Taiwan, 2019
108年度救國團青年獎章 - Selected work for Future Tech Exhibition “A novel potential therapeutic antibody to combat ALS and related neurodegenerative diseases”, 2018
技術團隊入選2018科技部未來科技展,作品”治療漸凍人及相關神經退化疾病的新穎抗體” - The 14th Young Investigator Award, TienTe Lee Biomedical Foundation, 2018
第14屆永信李天德青年醫藥科技獎 - Young Chemists Award of the Chemical Society, Taipei, 2016
2016年中國化學會傑出青年化學家獎章 - Ta-You Wu Memorial Award, 2015
104年度科技部吳大猷先生紀念獎 - TWAS Young Affiliate, East & Southeast Asia and Pacific Region, 2015-2019
2015第三世界科學院年輕學者成員(國際獎項) - The 13th Y.Z. Hsu Scientific Paper Award, 2015
第十三屆有庠科技論文獎 - Academia Sinica Research Award for Junior Research Investigators, 2015
104年度中研院年輕學者著作學獎 - Junior Faculty Award, the 12th International Conference on Alzheimer’s Disease and Parkinson’s Disease, 2015(國際獎項)
- Promising Women in Science Award, Wu Chieh Shiung Education Foundation, 2014
財團法人吳健雄學術基金會103年度台灣女科學家新秀獎 - Young Investigator Award, Biophysical Society of R.O.C., 2013
中華民國生物物理學會102年度傑出年輕學者獎 - Taiwan Dementia Society, LiFu Medical Research Foundation Academic Award, Advisor of the 1st Price, 2012, and 2nd Price, 2011
台灣臨床失智症學會財團法人立夫醫藥文教基金會學術獎第一名之指導教授2012及第 二名之指導教授2011 - The Taiwan Society for Biochemistry and Molecular Biology Traveling Fellowship, 2012
台灣生物化學及分子生物學會年輕學者出國研習優秀論文獎助2012 FAOBMB Congress - Academia Sinica Postdoctoral Fellowship, 2006-2007
RESEARCH HIGHLIGHTS:
- 2014/11/14 華視新聞雜誌專訪
- 2014/09/16 TDP-43蛋白球狀多聚體是一導致失憶症的關鍵分子
- 2012/10/12 帶負電的奈米金粒子可導正阿茲海默症關鍵蛋白的錯誤摺疊
RESEARCH INTERESTS:
Protein Folding/Misfolding, Amyloids, and Neurodegenerative Diseases
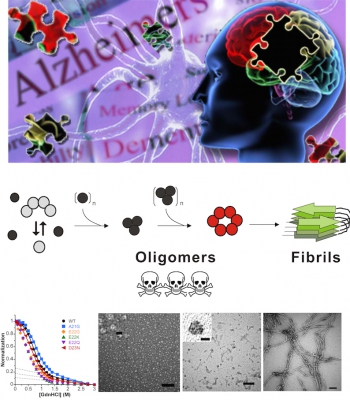
My research focuses on understanding the mechanism of protein misfolding diseases, amyloidosis, by various techniques including biochemical, biophysical, molecular, and cellular methods. Our long-term goal is to elucidate the disease mechanisms of amyloidosis in the aspects of protein folding and structure, pathogenic protein interactions, and relate the results to the medical consequences. We further utilize the knowledge to develop novel diagnostic means and therapeutic modalities. Many ageing-related neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) belong to amyloidosis. Among them, AD is the most serious problem in the 21st century. Amyloidosis is caused by aggregation of misfolded proteins to form amyloid fibrils comprising specific cross-β structures. Amyloid oligomers that exist in several neurodegenerative diseases imply a common toxicity mechanism in different neurodegenerative diseases. Currently, we are working on three amyloid and amyloid-like proteins and their interacting partners in neurodegenerative diseases. They are amyloid-β (Aβ) peptide and tau protein, the major substance in senile plaques and neurofibrillary tangles of AD patients respectively, α-synuclein, the component of Lewy bodies in PD, and TDP-43, a novel inclusion found in a subtype of frontotemporal lobar dementia (FTLD-U), amyotrophic lateral sclerosis (ALS), and AD. We start from elucidating the mechanism of such aggregation and further developing the diagnostic method, antibodies, and small molecule inhibitors. Moreover, we study the structure, function, and interactions of the related glycan conjugates, precursor proteins, and modifiers. The major research interests are listed as follows:
- Protein folding and misfolding of amyloids in neurodegenerative diseases.
- Amyloid protein oligomerization and the toxicity mechanisms in neurodegenerative diseases.
- Interactions of proteins, glycans, and lipids with the proteins involved in pathogenesis of the neurodegenerative diseases.
- Drug screening, diagnostic, and therapeutic developments in neurodegenerative diseases.
蛋白質錯誤摺疊及類澱粉蛋白疾病致病機轉
我們的研究重點在於運用多種生物化學、生物物理、及分子細胞學的技術,來了解蛋白質錯誤摺疊之類澱粉沉積症的致病機轉。類澱粉沉積症多與神經退化疾病相關,其中阿茲海默症為本世紀全球及臺灣日趨嚴重的疾病。類澱粉蛋白是由錯誤摺疊的蛋白質堆積,而形成擁有專一乙級結構的纖維。類澱粉蛋白的堆積會形成許多不同的形狀物,而對神經細胞有毒害。很有趣的是,許多有著不同一級氨基酸序列的類澱粉蛋白,也有著共同的形狀物,這暗示著它們享有共同的堆積機制及相關毒性。我們將從了解致病蛋白堆積物的功能及機制,進而發展其偵測方式、有效抗體、及小分子抑制物。同時,我們亦研究類澱粉蛋白之醣化修飾及其調節物之影響與交互作用。我們希望能提供這些神經退化疾病早期診斷與治療的新方向。目前我們的研究著重於下列類澱粉蛋白及其作用分子:(1)阿茲海默症病人腦部老年斑塊的主要組成:類澱粉乙形蛋白(amyloid-β)及神經糾結之tau蛋白、(2)帕金森氏症病人腦部路易士體的主要組成α-synuclein、及(3)額顳葉失憶/肌萎縮側索硬化症中病癥的TDP-43蛋白。我們主要的研究目標如下:
- 蛋白質在神經退化疾病中的摺疊與錯誤摺疊機制
- 類澱粉蛋白多倍體及在神經退化疾病中引發之毒性機制
- 類澱粉蛋白之醣化修飾及其調節物之影響與交互作用
- 針對神經退化疾病發展藥物篩選、診斷及治療策略
SELECTED PUBLICATIONS:
- Chia-Yi Ho, Yu-Jen Chang, Chih-Wen Yang, Orion Shih, U-Ser Jeng, Wan-Chen Huang, Ing-Shouh Hwang, Yun-Ru Chen*, accepted, “Membrane disruption properties of poly-glycine arginine dipeptide repeats affected by peptide repeats continuity and membrane composition”, Journal of Molecular Biology. (SCIE)
- Hsiang-Ting Lee, Han-Wen Chang, Yeh-Tung Lin, Shing- Jong Huang, Meng-Hsin Wu, Chang-Shun Tsai, Ling-Hsien Tu, Ming-Che Lee, Yun-Ru Chen*, and Jerry C. C. Chan*, accepted, “Oligomeric Protein Complexes formed by Beta Amyloid Peptides and Their Molecular Associates”, CHEMISTRY-A EUROPEAN JOURNAL. (SCIE)
- Gary Jen-Wei Chen§, Ming-Yun Chang§, Xin-Peng Lin, Debapriya Kundu, Yu-Jen Chang, and Yun-Ru Chen*, accepted, “Tau destabilization in a familial deletion mutant K280 accelerates its fibrillization and enhances the seeding effect”, JOURNAL OF BIOLOGICAL CHEMISTRY, 301(2), 108184. (SCIE)
- Ya-Ling Chiang, Yu-Jen Chang, Yun-Ru Chen, Ing-Shouh Hwang*, 2024, “Evidence Supporting Enrichment of Dissolved Air Gases near Hydrophobic Macromolecules in Aqueous Solutions”, Langmuir, 41(1), 285-291. (SCIE)
- Tien-Chang Lin‡, Orion Shih,b‡, Tien-Ying Tsai, Yi-Qi Yeh, Kuei-Fen Liao, Bradley W. Mansel, Ying-Jen Shiu, Chi-Fon Chang, An-Chung Su, Yun-Ru Chen*, and U-Ser Jeng*, 2024, “Binding structures of SERF1a with NT17-polyQ peptides of huntingtin exon 1 revealed by SECSWAXS, NMR and molecular simulation”, IUCrJ, 11(Pt 5), 849-858. (SCIE)
- Yu-Jen Chang, Kai-Tai Lin, Orion Shih, Chi-Hua Yang, Ching-Yu Chuang, Ming-Han Fang, Wei-Bin Lai, Yi-Chung Lee, Hung-Chih Kuo, Shang-Cheng Hung, Chi-Kuang Yao, U-Ser Jeng, Yun-Ru Chen*, 2024, “Sulfated disaccharide protects membrane and DNA damages from arginine-rich dipeptide repeats in ALS”, SCIENCE ADVANCES, 10, eadj0347. (SCIE)
- Tien-Ying Tsai, Wei-Ting Jhang, Hung-Kai Hsu, Yi-Tsu Chan, Chi-Fon Chang, Yun-Ru Chen*, 2024, “Amyloid Modifier SERF1a Accelerates Alzheimers Amyloid-β Fibrillization and Exacerbates the Cytotoxicity”, ACS Chemical Neuroscience, 15, 3,, 479-490. (SCIE)
- Chang, Yu-Jen; Chien, Yi-Hsin; Chang, Chieh-Chun; Wang, Pei-Ning; Chen, Yun-Ru*; Chang, Yun-Chorng*, 2024, “Detection of Femtomolar Amyloid-β Peptides for Early-Stage Identification of Alzheimer's Amyloid-β Aggregation with Functionalized Gold Nanoparticles”, ACS Applied Materials & Interfaces, 16, 3,, 3819-3828. (SCIE)
- Tien-Ying Tsai§, Chun-Yu Chen§, Tien-Wei Lin, Tien-Chang Lin, Feng-Lan Chiu, Orion Shih, Ming-Yun Chang, Yu-Chun Lin, An-Chung Su, U-Ser Jeng, Hung-Chih Kuo, Chi-Fon Chang, and Yun-Ru Chen*, 2023, “Amyloid Modifier SERF1a Interacts with PolyQ-expanded Huntingtin Exon 1 via Helical Interactions and Exacerbates PolyQ-induced Toxicity”, Communications Biology, 6, 767. (SCIE)
- Tien-Wei Lin, Jung-Kai Chang, Yih-Ru Wu, Tsung-Hsien Sun, Yang-Yu Cheng, Chien-Tai Ren, Mei-Hung Pan, Jin-Lin Wu, Kuo-Hsuan Chang, Hwai-I Yang, Chiung-Mei Chen*, Chung-Yi Wu*, and Yun-Ru Chen*, 2023, “Ganglioside-focused Glycan Array Reveals Abnormal Anti-GD1b Auto-antibody in Plasma of Preclinical Huntington’s Disease”, MOLECULAR NEUROBIOLOGY, 60(7), 3873-3882. (SCIE)
- Jin-Lin Wu and Yun-Ru Chen*, 2022, “Signal peptide stabilizes folding and inhibits misfolding of serum amyloid A”, PROTEIN SCIENCE, 31(12), e4485. (SCIE)
- Ming-Che Lee‡, Yi-Hung Liao‡, Shih-Hui Chen, and Yun-Ru Chen*., 2022, “Amyloid-β E22K Fibril in Familial Alzheimer’s Disease is More Thermo-stable and Susceptible to Seeding.”, IUBMB LIFE, 74(8), 739-747. (SCIE)
- Wan-Chin Chiang§, Yu-Sheng Fang§, Yuh-Shen Lye, Tzu-Yu Wong, Kiruthika Ganesan, Shih-Chieh Chou, Yun-Ru Chen*, 2022, “Hyperphosphorylation-mimetic TDP-43 Drives Amyloid Formation and Possesses Neuronal Toxicity at the Oligomeric Stage”, ACS Chemical Neuroscience, 37, 1,, 2599-2612. (SCIE)
- Jin-Lin Wu, Tung-Hung Su, Pei-Jer Chen, and Yun-Ru Chen*, 2022, “Acute‑phase serum amyloid A for early detection of hepatocellular carcinoma in cirrhotic patients with low AFP level”, Scientific Reports, 12, 5799. (SCIE)
- Yuh Shen Lye and Yun-Ru Chen*, 2022, “TAR DNA-binding protein 43 oligomers in physiology and pathology”, IUBMB LIFE, 2022, 1-18. (SCIE)
- Vu, Katrin; Lee, Ming-Che; Blankenburg, Gerhard; Chang, Yu-Jen; Chu, Ming-Lee; Erbe, Andreas; Lesser-Rojas, Leonardo; Chen, Yun-Ru; Chou, Chia-Fu*, 2021, “Time-evolved SERS signatures of DEP-trapped Aβ and Zn2+Aβ peptides revealed by a sub-10 nm electrode nanogap”, ANALYTICAL CHEMISTRY, 93(49), 16320-16329. (SCIE)
- Ya-Ling Chiang, Yu-Jen Chang, Yun-Ru Chen*, and Ing-Shouh Hwang*, 2021, “Effects of Dissolved Gases on Amyloid Fibril Morphology”, LANGMUIR, 37, 1,, 516-523. (SCIE)
- Yao-Hsiang Shih§, Ling-Hsien Tu§, Ting-Yu Chang, Kiruthika Ganesan, Wei-Wei Chang, Pao-Sheng Chang, Yu-Sheng Fang, Yeh-Tung Lin, Lee-Way Jin, and Yun-Ru Chen*, 2020, “TDP-43 interacts with amyloid-β, inhibits fibrillization, and worsens pathology in a model of Alzheimer’s disease”, NATURE COMMUNICATIONS, 11, 5950. (SCIE)
- Phillip Smethurst, Emmanuel Risse, Giulia E. Tyzack, Jamie S. Mitchell, Doaa M. Taha, Yun-Ru Chen, Jia Newcombe, John Collinge, Katie Sidle and Rickie Patani*, 2020, “Distinct responses of neurons and astrocytes to TDP-43 proteinopathy in amyotrophic lateral sclerosis”, BRAIN, 143(2), 430-440. (SCIE)
- Shih-Ling Huang, Lien-Szu Wu, Min Lee, Chin-Wen Chang, Wei-Cheng Cheng, Yu-Sheng Fang, Yun-Ru Chen, Pei-Lin Cheng, Che-Kun James Shen*, 2020, “A Robust TDP-43 Knock-In Mouse Model of ALS”, Acta Neuropathologica Communications, 8(1),3. (SCIE)
- Ling-Hsien Tu, Ning-Hsuan Tseng, Ya-Ru Tsai, Tien-Wei Lin, Yi-Wei Lo, Jien-Lin Charng, Hua-Ting Hsu, Yu-Sheng Chen, Rong-Jie Chen, Ying-Da Wu, Yi-Tsu Chan, Chang-Shi Chen, Jim-Min Fang,* and Yun-Ru Chen*, 2018, “Rationally Designed Divalent Caffeic Amide Inhibits Amyloid-β Fibrillization, Induce Fibril Dissociation, and Ameliorate Cytotoxicity”, EUROPEAN JOURNAL OF MEDICINAL CHEMISTRY, 158, 393-404. (SCIE)
- Chia-Jung Kuo, Hsu-Cheng Chiang, Chi-Ang Tseng, Chin-Fu Chang, Rajesh Kumar Ulaganathan, Tzu-Ting Ling, Yu-Jen Chang, Chiao-Chen Chen, Yun-Ru Chen, and Yit-Tsong Chen*., 2018, “A Lipid-Modified Graphene-Transistor Biosensor for Monitoring Amyloid-β Aggregation”, ACS APPLIED MATERIALS & INTERFACES, 10(15), 12311-12316. (SCIE)
- Ming-Che Lee, Wan-Cheng Yu, Yao-Hsiang Shih, Chun-Yu Chen, Zhong-Hong Guo, Shing-Jong Huang, Jerry C. C. Chan, and Yun-Ru Chen*, 2018, “Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease”, Scientific Reports, 8, 4772. (SCIE)
- Lin TW, Chang CF, Chang YJ, Liao YH, Yu HM, Chen YR*., 2017, “Alzheimer's amyloid-β A2T variant and its N-terminal peptides inhibit amyloid-β fibrillization and rescue the induced cytotoxicity”, PLOS ONE, 12(3), e0174561. (SCIE)
- Nguyen Quoc Thai, Ning-Hsuan Tseng, Mui Thi Vu, Tin Trung Nguyen, Huynh Quang Linh, Chin-Kun Hu*, Yun-Ru Chen*, and Mai Suan Li*, 2016, “Discovery of DNA dyes Hoechst 34580 and 33342 as good candidates for Alzheimer’s disease: in silico and in vitro study”, JOURNAL OF COMPUTER-AIDED MOLECULAR DESIGN, 30(8), 639-50. (SCIE)
- Yu-Jen Chang, Nguyen Hoang Linh, Hui-Ming Yu, Mai Suan Li*, and Yun-Ru Chen*, 2016, “Alzheimer’s Amyloid-β Sequesters Caspase-3 in vitro via its C-terminal Tail.”, ACS Chemical Neuroscience, 7(8), 1097-1106. (SCIE)
- Yu-Jen Chang, U-Ser Jeng, Ya-Ling Chiang, Ing-Shouh Hwang, and Yun-Ru Chen*, 2016, “Glycine-Alanine Dipeptide Repeat from C9orf72 Hexanucleotide Expansions Forms Toxic Amyloids Possessing Cell-to-cell Transmission Property”, Journal of Biological Chemistry, 291(10), 4903-4911. (SCIE)
- Chia‑Wei Lee, Lan‑Ling Jang, Huei‑Jyuan Pan, Yun‑Ru Chen, Chih‑Cheng Chen, and Chau‑Hwang Lee*, 2016, “Membrane roughness as a sensitive parameter reflecting the status of neuronal cells in response to chemical and nanoparticle treatments”, Journal of Nanobiotechnology, 14, 9. (SCIE)
- Patricia F. Kao, Yun-Ru Chen, Xiao-Bo Liu, Charles DeCarli, William W. Seeley, and Lee-Way Jin*, 2015, “Detection of TDP-43 oligomers in frontotemporal lobar degeneration-TDP”, ANNALS OF NEUROLOGY, 78(2), 211-221. (SCIE)
- Yu-Sheng Fang, Kuen-Jer Tsai, Yu-Jen Chang, Patricia Kao, Rima Woods, Pan-Hsien Kuo, Cheng-Chun Wu, Jhih-Ying Liao, Shih-Chieh Chou, Vinson Lin, Lee-Way Jin, Hanna S. Yuan, Irene H Cheng, Pang-Hsien Tu, and Yun-Ru Chen*, 2014, “Full-Length TDP-43 Forms Toxic Amyloid Oligomers that are Present in Frontotemporal Lobar Dementia-TDP Patients.”, Nature Communications, 5, 4824. (SCIE)
- Yu-Jen Chang, Yun-Ru Chen*, 2014, “The Co-existence of an Equal Amount of Alzheimer’s Amyloid-β 40 and 42 forms Structurally Stable and Toxic Oligomers through a Distinct Pathway”, FEBS JOURNAL, 281(11), 2674-2687. (SCIE)
- Huei-Jyuan Pan, Ruei-Lin Wang, Jian-Long Xiao, Yu-Jen Chang, Ji-Yen Cheng, Yun-Ru Chen, and Chau-Hwang Lee, 2014, “Using optical profilometry to characterize cell membrane roughness influenced by Amyloid-beta 42 aggregates and electric fields”, Journal of Biomedical Optics, 19(1), 011009. (SCIE)
- Man Hoang Viet, Chun-Yu Chen, Chin-Kun Hu, Yun-Ru Chen*, and Mai Suan Li*., 2013, “Discovery of Dihydrochalcone as potential lead for Alzheimer's disease: in silico and in vitro study”, PLoS One, 8(11), e79151. (SCIE)
- Rong-Jie Chen, Wei-Wei Chang, Yu-Chun Lin, Pei-Lin Pheng, Yun-Ru Chen*, 2013, “Alzheimer’s Amyloid-β Oligomers Rescue Cellular Prion Protein Induced Tau Reduction via Fyn Pathways”, ACS Chemical Neuroscience, 4(9), 1287-1296. (SCIE)
- Yi-Ting Wang, Pan-Hsien Kuo, Chien-Hao Chiang, Jhe-Ruei Liang, Yun-Ru Chen, Shuying Wang, James C. K. Shen, and Hanna S. Yuan*., 2013, “The truncated C-terminal RRM domain of TDP-43 plays a key role in forming proteinaceous aggregates.”, J Biol. Chem., 288(13), 9049-9057. (SCIE)
- Winny Ariesandi, Chi-Fon Chang, Tseng-Erh Chen,Yun-Ru Chen*, 2013, “Temperature-dependent structural changes of Parkinson's alpha-synuclein reveal the role of pre-existing oligomers in alpha-synuclein fibrillization”, PLoS One, 8(1), e53487. (SCIE)
- Yi-Hung Liao, Yu-Jen Chang, Yuji Yoshiike, Yun-Chorng Chang*, and Yun-Ru Chen*, 2012, “Negatively charged gold nanoparticles inhibit Alzheimer's amyloid-beta fibrillization, induce fibril dissociation, and mitigate neurotoxicity”, SMALL, 8(23), 3631-3639. (SCIE)
- Wei-Ting Chen, Chen-Jee Hong, Ya-Tzu Lin, Wen-Han Chang, He-Ting Huang, Jhih-Ying Liao, Chih-Ya Cheng, Hsiu-Chih Liu, Yun-Ru Chen*, and Irene H Cheng *, 2012, “Amyloid-beta (Abeta) D7H mutation increases oligomeric Abeta42 and alters properties of Abeta-zinc/copper assemblies ”, PLos One, 7(4), e35807. (SCIE)
- Chun-Lun Ni, Hoi-Ping Shi, Kuo-Ging Lin, Hui-Ming Yu, and Yun-Ru Chen*, 2011, “Folding Stability of Amyloid-beta 40 Monomer is an Important Determinant of the Nucleation Phase in Fibrillization”, Faseb Journal, 25(4),1390-1401. (SCIE)
- Wei-Ting Chen, Yi-Hung Liao, Hui-Ming Yu, Irene H. Cheng, and Yun-Ru Chen*, 2011, “Distinct Effects of Zn2+, Cu2+, Fe3+, and Al3+ on Amyloid-beta Stability, Oligomerization, and Aggregation: Amyloid-beta Destabilization Promotes Annular Protofibril Formation”, Journal of Biological Chemistry, 286(11), 9646-9656. (SCIE)
- Ni-Shian Lin, John Ching-Hao Chao, Fang-Chieh Chou, Chi-Fon Chang, Yun-Ru Chen, Yu-Jen Chang, Shing-Jong Huang, Wei-Hsiang Tseng, and Jerry C. C. Chan, 2010, “Molecular Structure of Amyloid Fibrils Formed by Residues 127 to 147 of the Human Prion Protein”, Chemistry - A European Journal, 16(18), 5492-5499. (SCIE)
- Yuji Yoshiike, Ryoichi Minai, Yo Matsuo, Yun-Ru Chen, Tetsuya Kimura, Akihiko Takashima* , 2008, “Amyloid Oligomer Conformation in a Group of Natively Folded Proteins”, PLos ONE., 3(9), e3235. (SCIE)
- Yun-Ru Chen and Charles G. Glabe*, 2006, “Distinct Early Folding and Aggregation Properties of Alzheimer Amyloid-b Peptide Aβ40 and Aβ42: Stable Trimer or Tetramer Formation by Ab42”, J Biol. Chem, 281, 24414-24422. (SCIE)
- Yun-Ru Chen and A. Clay Clark*, 2006, “Substitutions of prolines examine their role in kinetic trap formation of the caspase recruitment domain (CARD) of RICK”, Protein Science, 15, 395-409. (SCIE)
- Yun-Ru Chen and A. Clay Clark*, 2004, “Kinetic traps in the folding/unfolding of procaspase-1 CARD Domain”, Protein Science, 13, 2196-2206. (SCIE)
- Yun-Ru Chen and A. Clay Clark*, 2003, “Equilibrium and Kinetic Folding of the α-Helical Greek Key Protein Domain: Caspase Recruitment Domain (CARD) of RICK”, Biochemistry, 42, 6310-6320. (SCIE)
- C. Pop, Y. R. Chen, B. Smith, K. Bose, B. Bobay, A. Tripathy, S. Franzen and A. C. Clark*, 2001, “Removal of the pro-domain does not affect the conformation of the procaspase-3 dimer”, Biochemistry, 40, 14224-14235. (SCIE)