A research team led by Dr. Yun-Ru (Ruby) Chen at Genomic Research Center, aims to study the misfolded proteins related to neurodegenerative diseases. In 2014, the team reported that there are TDP-43 oligomers in frontotemporal lobar dementia (FTLD) patients and revealed the toxic role of TDP-43 oligomers(Research news: Curbing TDP-43 May Ease the Alzheimer’s Disease Progression) . The follow-up study was done by the team to find that TDP-43 oligomer worsens the pathology in Alzheimer’s disease (AD) model(Research news: Full-length TDP-43 Antibody Confirms Human FTLD). Together, these studies show that TDP-43 oligomers contribute to different neurodegenerative diseases.
Dr. Yun-Ru Chen observed that TDP-43 is hyperphosphorylated in FTLD patients. “This is interesting, there are some mechanisms underlying in TDP-43 hyperphosphorylation,” said Dr. Ruby. Based on this, their team started to explore the unknown mechanism.
Recently, the team led by Dr. Yun-Ru Chen published a research article in ACS Chemical Neuroscience, they found that the hyperphosphorylation of TDP-43 at the C-terminal promotes the formation of amyloid fibrils. In addition, this hyperphosphorylated TDP-43 can form a round TDP-43 oligomer and cause cellular toxicity. This article has also been selected as the supplementary cover page of the September issue of the journal.
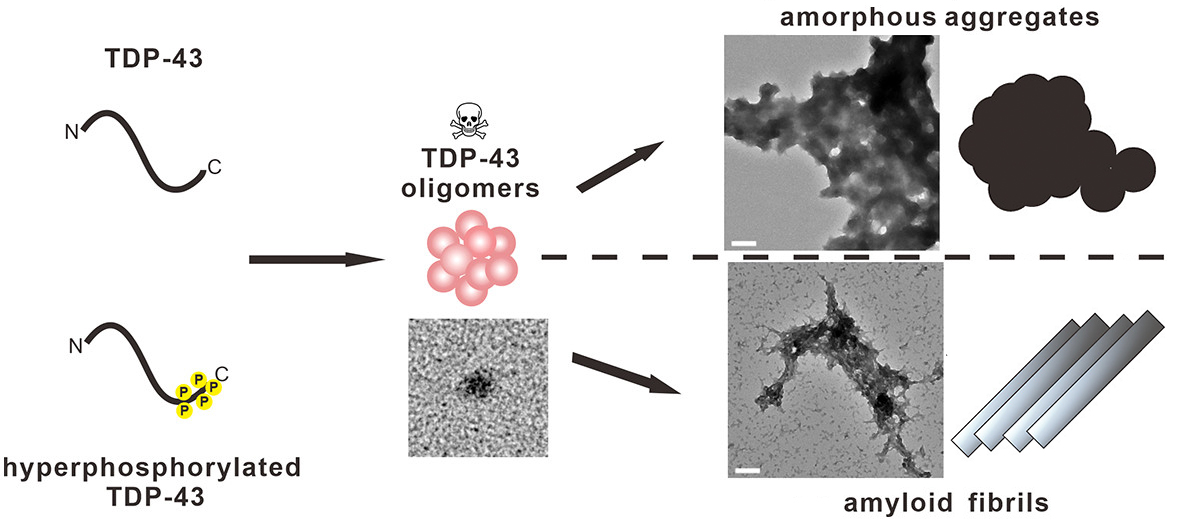 |
| Proposed aggregation and toxicity mechanism of TDP-43 and its hyperphosphorylation mimic |
In detail, the team firstly purified the TDP-43 variants which contain wild-type (WT), hyperphosphorylated (S5D), and nonphosphorylated (S5A) mimics. The S5D and S5A variants contain 5 serine mutations located at 379, 403, 404, 409, and 410 in the C-terminal to aspartate and to alanine, respectively.
They hypothesized whether hyperphosphorylation is the key factor in cell toxicity.
But, under the transmission electron microscope (TEM), the result revealed all the TDP-43 variants formed structurally similar spherical oligomers.
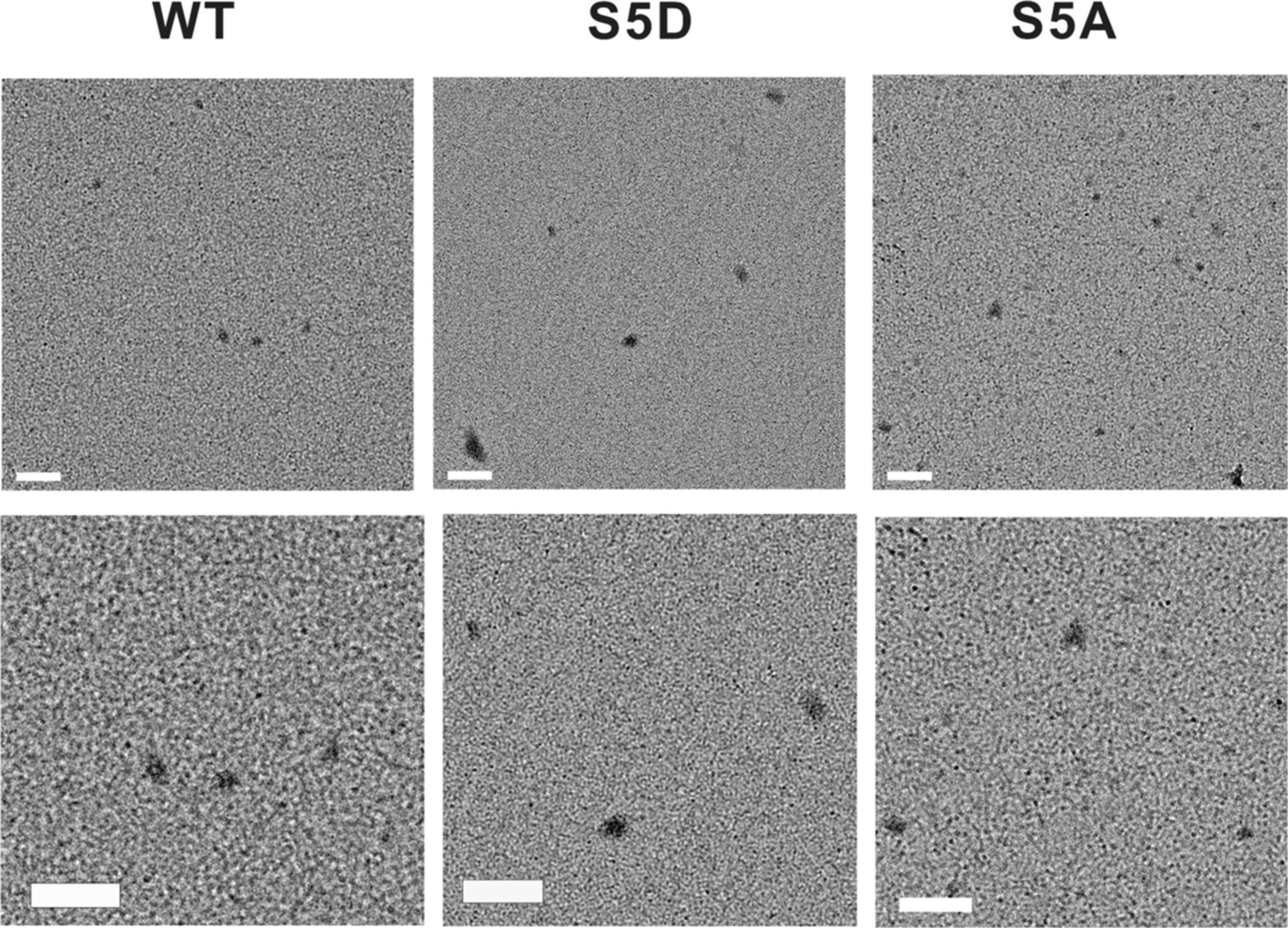 |
| TEM images of oligomers of TDP-43 variants. |
Next, the team found that the TDP-43 variants formed liquid-like condensates in which S5D is more soluble and dynamic than WT and S5A.
They suspected that S5D shares a common pathological feature with many neurogenerative diseases, as it can form cross-β sheet-rich amyloid fibrils, such as amyloid-β in Alzheimer’s disease. As a result, under the classical amyloid dye, Thioflavin-T assay, the team found that S5D exhibited classic amyloid fibrillization kinetics but not in WT and S5A.
Under TEM, they observed that the end-point products of WT and S5A formed a large cluster of irregular shape aggregates, which are named amorphous aggregates, but S5D formed some fibrils.
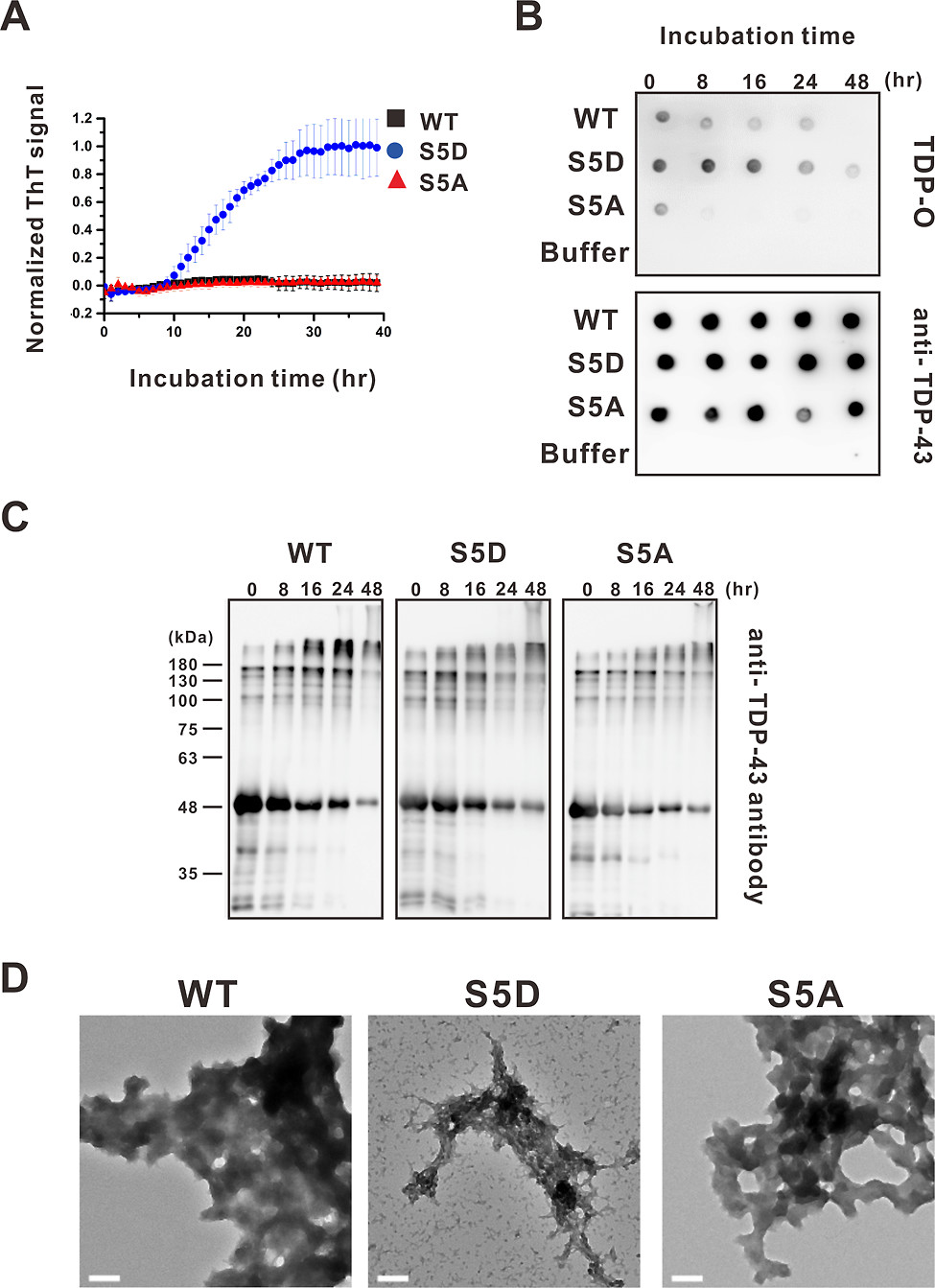 |
| Phosphor-mimic TDP-43 forms ThT-positive amyloid fibrils. |
It is known that the formation of TDP-43 oligomers is toxic to neurons. Are the final protein aggregates toxic? The answer is, No! The team used the primary neurons from rat and treated them with the oligomers and end-products of TDP-43 variants. They found that only TDP-43 oligomers are toxic to the neurons. This study is the first study to compare the toxicity of TDP-43 aggregates, and confirm that the TDP-43 oligomer is truly a neurotoxic species!
The charity event-Ice Bucket Challenge, which started in August 2014, and the movie The Theory of Everything was released in November of the same year, raising public awareness of amyotrophic lateral sclerosis. The topic of ALS has drawn public attention and discussion, but early diagnosis and therapeutic treatment have not yet been found. “However bad life may seem, while there is life, there is hope,”-said famous physicist Steven Hawking who was diagnosed with ALS. The team hopes to unearth more details and provides clues to the therapeutic development for TDP-43 proteinopathies.
 |
| Selected as Sub-cover pages of the September issue 2022 of ACS Chemical Neuroscience. Research Team (left to right): Research assistant, Ms Shih-Han Huang, Dr. Yun-Ru Chen, and PhD candidate Mr. Yuh Shen Lye |
The first authors Wan-Chin Chiang and Dr. Yu-Sheng Fang, former members of GRC colleagues. Second author Yuh Shen Lye was enrolled in the TIGP-Interdisciplinary Neuroscience program. Other assistance from GRC colleagues, Dr. Tzu-Yu Weng, Kiruthika Ganesan, Shih-Han Huang, Lan-Yun Chang, and Shih-Chieh Chou. Full research articles “Hyperphosphorylation-Mimetic TDP-43 Drives Amyloid Formation and Possesses Neuronal Toxicity at the Oligomeric Stage” can be found on the following website: https://pubs.acs.org/doi/full/10.1021/acschemneuro.1c00873