 Distinguished Professor
Distinguished Professor
schung
+886-2-27871279
+886-2-27871279
EDUCATION AND POSITIONS HELD:
- Ph.D., Chemistry, National Tsing Hua University, 1992
- Postdoctoral Fellow, University of California at Berkeley, 1994-1995
- Postdoctoral Fellow, The Scripps Research Institute, 1995-1998
- Assistant Research Fellow, Institute of Chemistry, Academia Sinica, 1998-2002
- Associate Research Fellow, Institute of Chemistry, Academia Sinica, 2002-2005
- Associate Professor, Department of Chemistry, National Tsing Hua University, 2005-2006
- Professor, Department of Chemistry, National Tsing Hua University, 2006-2007
- Distinguished Professor, Department of Chemistry, National Tsing Hua University, 2007-2009
- Professor, Genomics Research Center, Academia Sinica, 2009-2012
- Adjunct Professor, Department of Applied Chemistry, National Chiao Tung University, 2010-present
- Distinguished Professor, Genomics Research Center, Academia Sinica, 2012-present
- Director, Genomics Research Center, Academia Sinica, 2016-2022
- Acting Division Director of Biotechnology Incubation Center, Genomics Research Center, Academia Sinica, 2016-2018
HONORS:
- Postdoctoral Fellowship, Educational Ministry, 1994
- Young Scholar Research Publication Award, Academia Sinica, 2002
- Outstanding Young Investigator Award, The Chinese Chemical Society, 2002
- Outstanding Young Scholar Award, Tsing-Hua Fundation of Chemistry Technology, 2003
- Travel Grant Award for Young Chemists, IUPAC, 2003
- Mr. Tayou Wu Memorial Award, National Science Council, 2003
- Distinguished Research Award, National Science Council, 2004
- Outstanding Youth Medal, China Youth Corps, 2005
- Letcureship Award, International Conference on Cutting-Edge Organic Chemistry in Asia, 2006
- Yu-Ziang Hsu Scientific Paper Award, Far Eastern Y. Z. Hsu Science and Technology Memorial Foundation, 2008
- Distinguished Teaching Award, National Tsing Hua University, 2008
- Academic Publication Award, Chung-Shan Academic and Cultural Foundation, 2009
- Best Annual Article Award, The Chinese Chemical Society, 2009
- Distinguished Research Award, National Science Council, 2009-2011
- 17th Teco Award, Teco Technology Foundation, 2010
- Outstanding Scholar Chair, Foundation for the Advancement of Outstanding Scholarship (FAOS), 2010
- 7th Outstanding Biomedical Technology Award, Tien-Te Lee Biomedical Foundation, 2011
- 56th Academic Award, Ministry of Education, Taiwan, 2012
- Outstanding Research Award, Ministry of Science and Technology (3rd time), 2012
- Ho Chin Tui Outstanding Research Award, Ho Chin Tui Foundation, 2013
- 21st Research Grant Award, Mizutani Foundation for Glycoscience, Japan, 2014
- David Ginsburg Memorial Lectureship, Technion-Israel Institute of Technology, Haifa, Israel, 2014
- Investigator Award, Academia Sinica (2nd time), 2014
- Technique Transfer Award, Ministry of Science and Technology, 2015
- Academic Summit Program Award, Ministry of Science and Technology, 2016
- Academic Award, The Chinese Chemical Society at Taipei (CSLT), 2016
- 13th National Innovation Award (Academic Research Category), Institute for Biotechnology and Medicine Industry, 2016
- Outstanding Merit Award, Wang Ming-Ning Memorial Foundation, 2017
- Excellent Research Project of Future Tech Exhibition, Ministry of Science and Technology, 2017
- Academic Summit Program Award, Ministry of Science and Technology (2nd time), 2021
- Future Tech Award, Ministry of Science and Technology, 2021
- Academician, Academia Sinica, 2024
RESEARCH INTERESTS:
Carbohydrate Synthesis, Glycotechnology, and Glycobiology
The research in Dr. Hung’s group aims on the development of novel methodologies for the synthesis of biologically potent oligosaccharides, glycolipids, and glycoproteins via a combination of regioselective one-pot protection and stereoselective one-pot glycosylation. The major interests include five topics:
- Discovery of new technologies for carbohydrate synthesis
- Preparation of cell-surfaced carbohydrates (heparin/heparan sulfate, GPI anchors) and mycobacterial cell envelope components
- Development of heparin-related microarrays, dendrimers, and nanoparticles
- Study of heparin-protein interaction
- Investigation of carbohydrate-based anti-tuberculosis vaccines
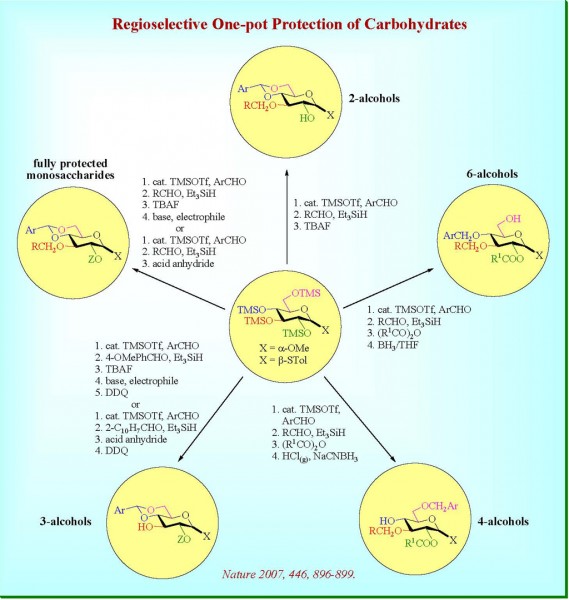 |
| Regioselective One-pot Protection of Carbohydrates |
SELECTED PUBLICATIONS:
- Byung-sun Jeon, Teng-Yi Huang, Mark W. Ruszczycky, Sei-hyun Choi, Namho Kim, Joseph Livy Franklin, Shang-Cheng Hung*, Hung-wen Liu*, 2022, “Byproduct formation during the biosynthesis of spinosyn A and evidence for an enzymatic interplay to prevent its formation”, TETRAHEDRON, 103, 132569. (SCIE)
- Jan JT, Cheng TR, Juang YP, Ma HH, Wu YT, Yang WB, Cheng CW, Chen X, Chou TH, Shie JJ, Cheng WC, Chein RJ, Mao SS, Liang PH, Ma C*, Hung SC*, Wong CH*, 2021, “Identification of existing pharmaceuticals and herbal medicines as inhibitors of SARS-CoV-2 infection.”, Proceedings of the National Academy of Sciences of the United States of America, 118(5). (SCIE, A&HCI, Scopus, EI, TSSCI, THCI)
- Gannedi V, Villuri BK, Reddy SN, Ku CC, Wong CH*, Hung SC*, 2021, “Practical Remdesivir Synthesis through One-Pot Organocatalyzed Asymmetric (S)-P-Phosphoramidation.”, The Journal of Organic Chemistry, 86(7), 4977-4985. (SCIE)
- Chiu LT, Sabbavarapu NM, Lin WC, Fan CY, Wu CC, Cheng TR, Wong CH*, Hung SC*, 2020, “Trisaccharide Sulfate and Its Sulfonamide as an Effective Substrate and Inhibitor of Human Endo-O-sulfatase-1.”, Journal of the American Chemical Society, 142(11), 5282-5292. (SCIE)
- Sakamoto K, Ozaki T, Ko YC, Tsai CF, Gong Y, Morozumi M, Ishikawa Y, Uchimura K, Nadanaka S, Kitagawa H, Zulueta MML, Bandaru A, Tamura JI, Hung SC, Kadomatsu K, 2019, “Glycan sulfation patterns define autophagy flux at axon tip via PTPRsigma-cortactin axis.”, Nature Chemical Biology, 15(7), 699-709. (SCIE)
- Yeh, C.-J.; Ku, C.-C.; Lin, W.-C.; Fan, C.-Y.; Medel Manuel L. Zulueta, M. M. L.; Manabe, Y.; Fukase, K.; Li, Y.-K.; Hung, S.-C.*, 2019, “Single-Step Per-O-Sulfonation of Sugar Oligomers with Concomitant 1,6-Anhydro Bridge Formation for Binding Fibroblast Growth Factors”, CHEMBIOCHEM, 20, 237-240. (SCIE)
- Wang, C.-C.*; Sabbavarapu, N. M.; Podilapu, A. R.; Liao, P.-H.; Hung, S.-C.*, 2018, “One-Pot Protection, Glycosylation and Protection-Glycosylation Strategies of Carbohydrates”, Chem. Rev., 118, 8025-8104. (SCIE)
- Zulueta, M. M. L.*; Chyan, C.-L.; Hung, S.-C.*, 2018, “Structural Analysis of Synthetic Heparan Sulfate Oligosaccharides with Fibroblast Growth Factors and Heparin-Binding Hemagglutinin”, Curr. Opin. Struct. Biol., 50, 126-133.
- Ho, K.-M.; Zulueta, M. M. L.; Hung, S.-C.*, 2017, “Stereoselective One-pot Synthesis of Polypropionates”, Nat. Commun, 8, 679. (SCIE)
- Huang, T.-Y.; Irene, D.; Ku, C.-C.; Zulueta, M. M. L.; Tai, T.-J.; Lain, S.-H.; Cheng, C.-P.; Tsai, P.-X.; Lin, S.-Y.; Chen, Z.-G.; Hsiao, C.-D.; Chyan, C.-L.*; Hung, S.-C.*, 2017, “Structure of the Complex between a Heparan Sulfate Octasaccharide and Mycobacterial Heparin-Binding Hemagglutinin”, Angew. Chem. Int. Ed, 56, 4192-4196.
- Tsai, C.-T.; Zulueta, M. M. L.; Hung, S.-C.*, 2017, “Synthetic Heparin and Heparan Sulfate: Probes in Defining Biological Functions”, Curr. Opin. Chem. Biol., 21, 152-159. (SCIE)
- Padiyar, L. T.; Zulueta, M. M. L.; Sabbavarapu, N. M.; Hung, S.-C.*, 2017, “Yb(OTf)3-Catalyzed Desymmetrization of myo-Inositol-1,3,5-Orthoformate and Its Application in the Synthesis of Chiral Inositol Phosphates”, J. Org. Chem, 82, 11418-11430. (SCIE)
- Stewart, K. L.; Hughes, E.; Yates, E. A.; Huang, T.-Y.; Lima, M. A.; Rudd, T. R.; Guerrini, M.; Hung, S.-C.*; Radford, S. E.*; Middleton, D. A.*, 2016, “Atomic Details of the Interactions of Glycosaminoglycans with Amyloid-β Fibrils”, J. Am. Chem. Soc., 138, 8328-8331. (SCIE)
- Liang, W. G.; Huang, T.-Y.; Zulueta, M. M. L.; Hung, S.-C.*; Tang, W.-J.*, 2016, “Structural Basis for Oligomerization and Glycosaminoglycan-Binding of CCL5 and CCL3”, Proc. Natl. Acad. Sci. USA, 113, 5000-5005.
- Ho, G.-M.; Huang, C.-J., Li, E. Y.-T.*, Hsu, S.-K.; Wu, T.; Zulueta, M. M. L.; Wu, K. P.-C.; Hung, S.-C.*, 2016, “Unconventional exo-Selectivity in Thermal Normal-Electron-Demand Diels–Alder Reactions”, Sci. Rep, 6, 35147.
- Kuwabara K; Nishitsuji K; Uchimura K; Hung SC; Mizuguchi M; Nakajima H; Mikawa S; Kobayashi N; Saito H; Sakashita N., 2015, “Cellular interaction and cytotoxicity of the Iowa mutation of apolipoprotein A-I (ApoA-IIowa) amyloid mediated by sulfate moieties of heparan sulfate.”, JOURNAL OF BIOLOGICAL CHEMISTRY, 290, 24210-24221. (SCIE)
- Medel Manuel L. Zulueta*, Donala Janreddy and Shang-Cheng Hung*, 2015, “One-Pot Methods for the Protection and Assembly of Sugars”, ISRAEL JOURNAL OF CHEMISTRY, 55(3), 347-359. (SCIE)
- Patil PS; Cheng TJR; Zulueta MM; Yang ST; Lico LS; Hung SC.*, 2015, “Total synthesis of tetraacylated phosphatidylinositol hexamannoside and evaluation of its immunomodulatory activity.”, Nat Commun., 6, 7239. (SCIE)
- Zulueta, M. M. L.; Hung, S.-C.*, 2015, Glycoscience: Biology and Medicine, 365-371 pages, Tokyo: Springer.
- Ko YC; Tsai CF; Wang CC*; Dhurandhare VM; Hu PL; Su TY; Lico LS; Zulueta MM; Hung SC*, 2014, “Microwave-assisted one-pot synthesis of 1,6-anhydrosugars and orthogonally protected thioglycosides.”, J Am Chem Soc., 136, 14425-14431. (SCIE)
- Chang CH; Lico LS; Huang TY; Lin SY; Chang CL; Arco SD; Hung SC.*, 2014, “Synthesis of the heparin-based anticoagulant drug Fondaparinux.”, ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 53, 9876-9879. (SCIE)
- Li YC; Ho IH; Ku CC; Zhong YQ; Hu YP; Chen ZG; Chen CY; Lin WC; Zulueta MM; Hung SC*; Lin MG; Wang CC; Hsiao CD*, 2014, “Interactions That Influence the Binding of Synthetic Heparan Sulfate Based Disaccharides to Fibroblast Growth Factor-2”, ACS Chem Biol., 9, 1912-1717. (SCIE)
- Hsu Y; Ma HH; Lico LS; Jan JT; Fukase K; Uchinashi Y; Zulueta MM; Hung SC.*, 2014, “One-pot synthesis of N-acetyl- and N-glycolylneuraminic acid capped trisaccharides and evaluation of their influenza A(H1N1) inhibition.”, ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 53, 2413-2416. (SCIE)
- Patil, P. S.; Zulueta, M. M. L.; Hung, S.-C., 2014, “Synthesis of Phosphatidylinositol Mannosides”, Journal of the Chinese Chemical Society, 61, 151-162. (SCIE)
- Huang, T.-Y.; Zulueta, M. M. L.; Hung, S.-C., 2014, “Regioselective One-pot Protection, Protection-Glycosylation and Protection- Glycosylation-Glycosylation of Carbohydrates: A Case Study with D-Glucose”, Organic & Biomolecular Chemistry, 12(2), 376-382. (SCIE)
- Zulueta, M. M. L.; Lin, S.-Y.; Hung, S.-C., 2013, “Chemical Synthesis of Oligosaccharides Based on Heparin and Heparan Sulfate”, Trends in Glycoscience and Glycotechnology, 25, 141-158. (SCIE)
- Patil, P. S.; Lee, C.-C.; Huang, Y.-W.; Zulueta, M. M. L.; Hung, S.-C., 2013, “Regioselective and Stereoselective Benzylidene Installation and One-pot Protection of D-mannose”, Organic & Biomolecular Chemistry, 11(16), 2605-2612. (SCIE)
- Lu, F.-C.; Lico, L. S.; Hung, S.-C., 2013, “Synthesis of Fluorogenic Substrate for α-L-Iduronidase”, ARKIVOC, 2013(2), 13-21. (SCIE, Science Citation Index Expande)
- Zulueta, M. M. L.; Zhong, Y.-Q.; Hung, S.-C., 2013, “Synthesis of Rare L-Hexoses and Their Related Biomolecules”, Chemical Communications, 49(32), 3275-3287. (SCIE)
- Woolfson, D. N.; Hung, S.-C., 2013, “Synthetic Biomolecules”, Current Opinion in Chemical Biology, 17(6), 925-928. (SCIE)
- Zulueta, M. M. L.; Lin, S.-Y.; Hu, Y.-P.; Hung, S.-C., 2013, “Synthetic Heparin and Heparan Sulfate Oligosaccharides and their Protein Interactions”, Current Opinion in Chemical Biology, 17(6), 1023-1029. (SCIE)
- Hu, Y.-P.; Zhong, Y.-Q.; Chen, Z.-G.; Chen, C.-Y.; Shi, Z.; Zulueta, M. M. L.; Ku, C.-C.; Lee, P.-Y.; Wang, C.-C.; Hung, S.-C., 2012, “Divergent Synthesis of 48 Heparan Sulfate-Based Disaccharides and Probing the Specific Sugar–Fibroblast Growth Factor-1 Interaction”, J. Am. Chem. Soc., 134(51), 20722-20727. (SCIE)
- Zulueta, M. M. L.; Lin, S.-Y.; Lin, Y.-T.; Huang, C.-J.; Wang, C.-C.; Ku, C.-C.; Shi, Z.; Chyan, C.-L.; Irene, D.; Lim, L.-H.; Tsai, T.-I; Hu, Y.-P.; Liu, J.-Y.; Chang, W.; Arco, S. D.; Wong, C.-H.; Hung, S.-C., 2012, “α-Glycosylation by D-Glucosamine-Derived Donors: Synthesis of Heparosan and Heparin Analogs that Interact with Mycobacterial Heparin-Binding Hemagglutinin”, J. Am. Chem. Soc., 134(21), 8988-8995. (SCIE)
- Hsu, Y.; Lu, X.-A.; Zulueta, M. M. L.; Tsai, C.-M.; Lin, K.-I; Hung, S.-C.; Wong, C.-H., 2012, “Acyl and Silyl Group Effects in Reactivity-based One-pot Glycosylation: Synthesis of Embryonic Stem Cell Surface Carbohydrates Lc4 and IV2Fuc-Lc4”, J. Am. Chem. Soc., 134, 4549-4552. (SCIE)
- Luo, S.-Y.; Tripathi, A.; Zulueta, M. M. L.; Hung, S.-C., 2012, “2-Allylphenyl Glycosides as Glycosyl Donors for Sugar Coupling”, Carbohydrate Research, 352, 197-201. (SCIE)
- Hsu, C.-Y.; Lee, I-C.; Lico, L. S.; Uang, B.-J.; Hung, S.-C., 2012, “Synthesis of a Furanosyl-pyranone Derivative Related to the Tri-O-heterocyclic Core of Herbicidins”, JOURNAL OF THE CHINESE CHEMICAL SOCIETY, 59, 421-425. (SCIE)
- Hung, S.-C.; Lu, X.-A.; Lee, J.-C.; Fang, S.-L.; Fan, T.-C.; Chang, M. D.-T.; Zulueta, M. M. L.; Chiu, L.-T., 2012, “Synthesis of Heparin Oligosaccharides and Their Interaction with Eosinophil-Derived Neurotoxin”, Org. Biomol. Chem., 10, 760-772. (SCIE)
- Hsu, C.-H.; Hung, S.-C.; Wu, C.-Y.; Wong, C.-H., 2011, “Toward Automatic Oligosaccharide Synthesis”, Angewandte Chemie-International Edition, 50(50), 11872-11923. (SCIE)
- Chung, C.-C.; Zulueta, M. M. L.; Padiyar, L. T.; Hung, S.-C., 2011, “Desymmetrization of 2,4,5,6-Tetra-O-benzyl-D-myo-inositol for the Synthesis of Mycothiol”, Org. Lett., 13, 5496–5499. (SCIE)
- Lee, I.-C.; Zulueta, M. M. L.; Shie, C.-R.; Arco, S. D.; Hung, S.-C., 2011, “Deuterium-Isotope Study on the Regioselective Ring Opening of Benzylidene Acetals”, Org. Biomol. Chem., 9, 7655–7658. (SCIE)
- Huang, T.-Y.; Zulueta, M. M. L.; Hung, S.-C., 2011, “One-Pot Strategies for the Synthesis of the Tetrasaccharide Linkage Region of Proteoglycans”, Org. Lett., 13, 1506–1509. (SCIE)
- Wang, C.-C.; Zulueta, M. M. L.; Hung, S.-C., 2011, “Regioselective One-pot Protection and Protection-glycosylation of Carbohydrates”, Chimia, 65, 54-58. (SCIE)
- Hu, Y.-P.; Lin, S.-Y.; Huang, C.-Y.; Zulueta, M. M. L.; Liu, J.-Y.; Chang, W.; Hung, S.-C., 2011, “Synthesis of 3-O-Sulfonated Cell-Surface Heparan Sulfate Octasaccharides that Inhibit Herpes Simplex Virus Type 1 Host-Cell Interaction”, Nature Chemistry, 3, 557–563. (SCIE)
- Wang, C.-C.; Kulkarni, S. S.; Zulueta, M. M. L.; Hung, S.-C., 2011, “Synthesis of Hemagglutinin-Binding Trisaccharides”, Advances in Experimental Medicine and Biology, 705, 691-726. (SCIE)
- Chang, K.-L.; Zulueta, M. M. L.; Lu, X.-A.; Zhong, Y.-Q.; Hung, S.-C., 2010, “Regioselective One-pot Protection of D-Glucosamine”, J. Org. Chem, 75, 7424-7427. (SCIE)
- Patil, P. S.; Hung, S.-C., 2010, “Synthesis of Mycobacterial Triacylated Phosphatidylinositol Dimannoside Containing an Acyl Lipid Chain at 3-O of Inositol”, Org. Lett., 12, 2618-2621. (SCIE)
- Padiyar, L. T.; Wen, Y.-S.; Hung, S.-C., 2010, “Metal Trifluromethanesulfonate-Catalyzed Regioselective Acylation of myo-Inositol Orthoformate”, Chem. Commun., 46, 5524-5526. (SCIE)
- Hung, S.-C., 2010, “Synthesis of Mycobacterial Cell Envelope Components”, Natural Sciences Newsletter, 22, 43-46.
- Shie, C.-R.; Tzeng, Z.-H.; Wang, C.-C.; Hung, S.-C. , 2009, “Metal Trifluoromethanesulfonate-Catalyzed Regioselective Reductive Ring Opening of Benzylidene Acetals”, Journal of the Chinese Chemical Society, 56, 510–523. (SCIE)
- Chi, F.-C.; Kulkarni, S. S.; Zulueta, M. M. L.; Hung, S.-C. , 2009, “Synthesis of Alginate Oligosaccharides Containing L-Guluronic Acids”, Chemistry-An Asian Journal, 4, 386–390. (SCIE)
- Patil, P. S.; Hung, S.-C., 2009, “Total Synthesis of Phosphatidylinositol Dimmanoside: A Cell-Envelope Component of Mycobacterium tuberculosis”, Chemistry-A European Journal, 15, 1091–1094. (SCIE)
- Luo, S.-Y.; Jang, Y.-J.; Liu, J.-Y.; Chu, C.-S.; Liao, C.-C.; Hung, S.-C., 2008, “Carbohydrates-Templated Asymmetric Diels-Alder Reactions of Masked ortho-Benzoquinones for the Synthesis of Chiral Bicyclo[2.2.2]oct-5-en-2-ones”, Angewandte Chemie-International Edition, 47, 8082–8085. (SCIE)
- Fan, T.-C.; Fang, S.-L.; Hwang, C.-S.; Hsu, C.-Y.; Lu, X.-A.; Hung, S.-C.; Lin, S.-C.; Chang, M. D.-T. , 2008, “Characterization of Molecular Interactions between Eosinophil Cationic Protein and Heparin”, J. Biol. Chem., 283, 25468–25474. (SCIE)
- Wang, C.-C.; Kulkarni, S. S.; Lee, J.-C.; Luo, S.-Y.; Hung, S.-C. , 2008, “Regioselective One-pot Protection of Glucose”, Nat. Protoc, 3, 97–113. (SCIE)
- Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Kulkarni, S. S.; Huang, Y.-W.; Lee, C.-C.; Chang, K.-L.; Hung, S.-C., 2007, “Regioselective One-pot Protection of Carbohydrates”, Nature, 446, 896–899. (SCIE)
- Wang, C.-C.; Kulkarni, S. S.; Hung, S.-C., 2007, “Regioselective One-pot Protection of Carbohydrates”, Synform, 3, A32–A34.
- Luo, S.-Y.; Kulkarni, S. S.; Chou, C.-H.; Liao, W.-M.; Hung, S.-C., 2006, “A Concise Synthesis of Tetrahydroxy-LCB, -Galactosyl Ceramide, and 1,4-Dideoxy-1,4-imino-L-ribitol via D-Allosamines as Key Building Blocks”, J. Org. Chem., 71, 1226–1229. (SCIE)
- Lu, L.-D.; Shie, C.-R.; Kulkarni, S. S.; Pan, G.-R.; Lu, X.-A.; Hung, S.-C., 2006, “Synthesis of 48 Disaccharide Building Blocks for the Assembly of a Heparin and Heparan Sulfate Oligosaccharide Library”, Org. Lett, 8, 5995–5998. (SCIE)
- Shie, C.-R.; Tzeng, Z.-H.; Kulkarni, S. S.; Uang, B.-J.; Hsu, C.-Y.; Hung, S.-C. , 2005, “Cu(OTf)2 as an Efficient and Dual-Purpose Catalyst in the Regioselective Reductive Ring Opening of Benzylidene Acetals”, Angewandte Chemie-International Edition, 44, 1665–1668. (SCIE)
- Kulkarni, S. S.; Liu, Y.-H.; Hung, S.-C., 2005, “Neighboring Group Participation of 9-Anthracenylmethyl Group in Glycosylation: Preparation of Unusual C-Glycosides”, J. Org. Chem., 70, 2808–2811. (SCIE)
- Lee, J.-C.; Chang, S.-W.; Liao, C.-C.; Chi, F.-C.; Chen, C.-S.; Wen, Y.-S.; Wang, C.-C.; Kulkarni, S. S.; Puranik, R.; Liu, Y.-H.; Hung, S.-C., 2004, “From D-Glucose to Biologically Potent L-Hexoses: Synthesis of -L-Iduronidase Fluorogenic Detector and the Disaccharide Moieties of Bleomycin A2 and Heparan Sulfate”, Chemistry-A European Journal, 10, 399–415. (SCIE)
- Chou, C. H., Wu, C. S., Chen, C. H., Lu, L. D., Kulkarni, S. S., Wong, C. H. and Hung, S. C., 2004, “Regioselective glycosylation of neamine core: a facile entry to kanamycin B related analogues.”, Org Lett, 6(4), 585-588. (SCIE)
- Lee, C.-J.; Lu, X.-A.; Kulkarni, S. S.; Wen, Y.-S.; Hung, S.-C., 2004, “Synthesis of Heparin Oligosaccharides”, J. Am. Chem. Soc., 126, 476–477. (SCIE)
- Tai, C.-A.; Kulkarni, S. S.; Hung, S.-C. , 2003, “Facile Cu(OTf)2-catalyzed Preparation of Per-O-acetylated Hexopyranoses with Stoichiometric Acetic Anhydride and Sequential One-pot Anomeric Substitution to Thioglycosides under Solvent-free Conditions”, J. Org. Chem., 68, 8719–8722. (SCIE)
- Hung, S.-C.; Wen, Y.-F.; Chang, J.-W.; Liao, C.-C.; Uang, B.-J., 2002, “A Highly Diastereoselective Synthesis of (1R)-(+)-Camphor Based Chiral Allenes and their Asymmetric Hydroboration-Oxidation Reactions”, J. Org. Chem., 67, 1308–1313. (SCIE)
- Wang, C.-C.; Luo, S.-Y.; Shie, C.-R.; Hung, S.-C., 2002, “Metal Trifluoromethanesulfonate-Catalyzed Regioselective Borane-Reductive Ring Opening of Benzylidene Acetals: A Concise Synthesis of 1,4-Dideoxy-1,4-imino-L- xylitol”, Org. Lett., 4, 847–849. (SCIE)
- Lin, T.-S.; Chia, C.-M.; Hsiao, J.-C.; Chang, W.; Ku, C.-C.; Hung, S.-C.; Tzou, D.-L. M., 2002, “Structural analysis of the extracellular domain of vaccinia virus envelope protein, A27L, by NMR and CD spectroscopy”, J. Biol. Chem., 277, 20949–20959. (SCIE)
- Wang, C.-C.; Lee, J.-C.; Luo, S.-Y.; Fan, H.-F.; Pai, C.-L.; Yang, W.-C.; Lu, L.-D.; Hung, S.-C., 2002, “Synthesis of Biologically Potent 12 Linked Disaccharide Derivatives via Regioselective One-pot Protection-glycosylation”, Angewandte Chemie-International Edition, 41, 2360–2362. (SCIE)
- Hung, S.-C.; Thopate, S. R.; Chi, F.-C.; Chang, S.-W.; Lee, J.-C.; Wang, C.-C.; Wen, Y.-S., 2001, “1,6-Anhydro--L-hexopyranoses as Potent Synthons in the Synthesis of the Disaccharide Units of Bleomycin A2 and Heparin”, J. Am. Chem. Soc., 123, 3153–3154. (SCIE)
