Magnetic resonance imaging (MRI) plays a pivotal role in the medical care of patients with or at risk for hepatocellular carcinoma (HCC). Clinically, there exists about a 35% chance that contrast agents will be needed to improve the image sensitivity of MRI examinations. Commonly used products are iron-based contrast agents and gadolinium-based contrast agents.
Since liver tissue actively absorbs iron ions, detecting HCC with a general iron-based contrast agent is difficult. And considering gadolinium can stay in the body for months to years after receiving these drugs, it is an urge to create novel nanocomposites for enhancing the noise-signal ratio of MRI to improve the diagnosis accuracy for tumors.
The team led by Dr. Michael Hsiao of Genomics Research Center developed a functionalized montmorillonite (MMT) material that can be used as a magnetic composite material to carry FePt NPs and optimized for MRI-related applications. Moreover, porous MMT layers can be used as a drug-loading platform and magnetic fluid hyperthermia (MFH) inducer. Their study was published in the October 9, 2021 issue of the Journal of Nanobiotechnology.
Through the collaborative work, Dr. Ming-Hsien Chan, the article’s first author, and Dr. Da-Hua Wei of KMU chose montmorillonite (MMT) as a magnetic composite material for absorbing FePt NPs. They use CTAB to modify MMT to form a layered structure. The final sheet-like MMT is then coated with FePt NPs surface to give it paramagnetic properties.
The layer structure can limit the space of the magnetic material and optimize magnetic efficiency. The researchers compared the contrast performance; the Ms of optimized FePt@MMT nanocomposites (FePt@MMT/10.5%) increased to 24.54 emu/g (175% higher than FePt), which can be applied for achieving in situ MRI diagnosis.
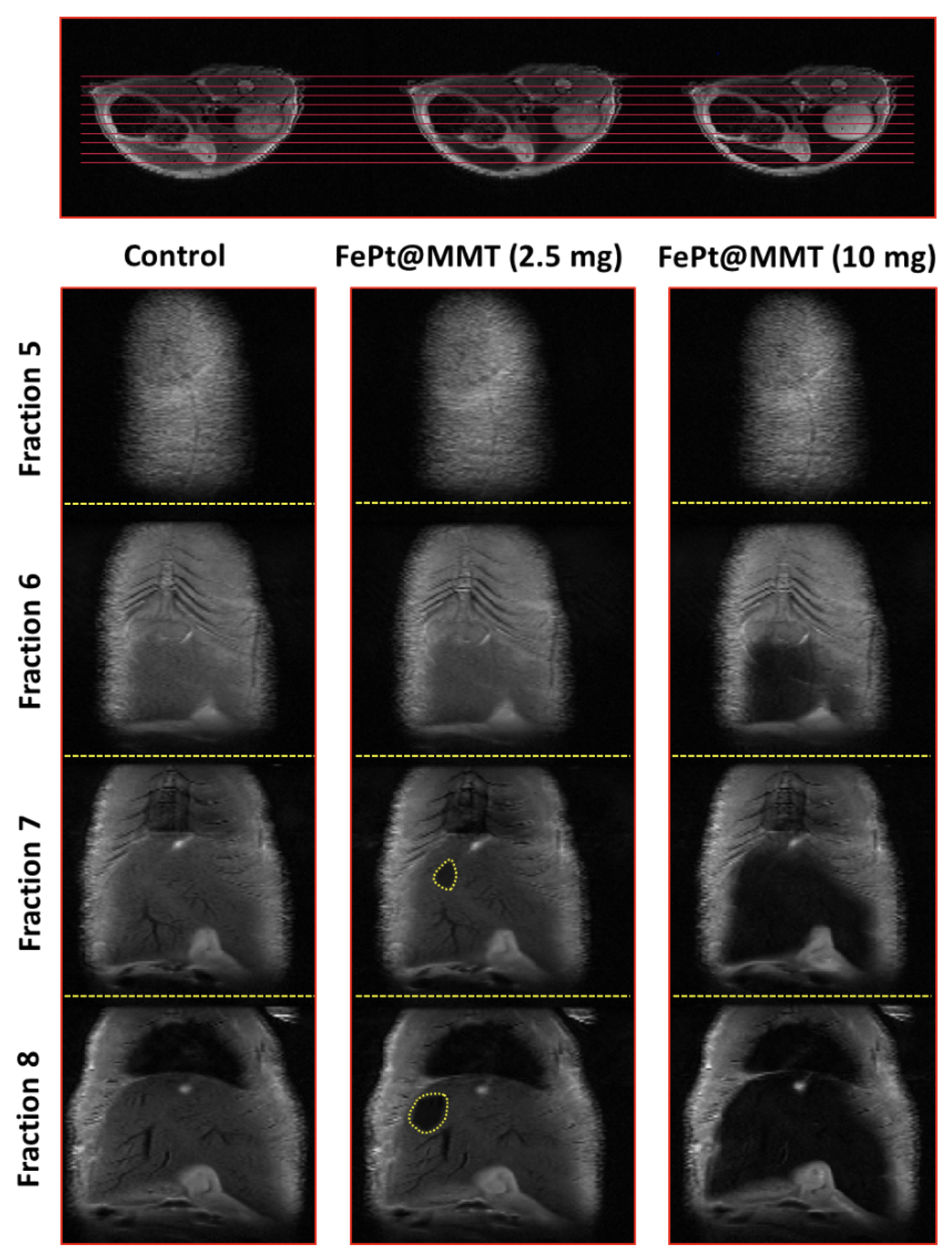
After applying FePt@MMT to the HCC mouse, the T2-weighted MRI signal of the orthotopic HCC tumor became darker. Before injection, the appearance of the tumor area could not be observed under an MRI analysis. These excellent properties of the FePt@MMT allowed the researchers to keep the HCC tumor, which is hardly visible with current MRI contrast agents.
In addition to having higher resolution, porous MMT layers can simultaneously be used as a drug-loading platform and capture chemotherapeutic drugs, such as mitoxantrone (MIT), to suppress central tumor growth.
“We used extra magnetic guidance to help FePt@MMT-MIT accumulate in SKHep1 liver cancer cells, indicating that magnetic induction of MIT drug accumulation can be used as a cumulative drug in specific tissues or a targeted approach for target locations,” says study co-author Chi-Long Chen, M.D, Department of Pathology at TMUH.
Not only was FePt@MMT able to deliver drugs, but the heating response of hydrophilic FePt@MMT nanocomposites was also demonstrated that this nanocomposite could raise the water to 60 degrees, thereby killing cancer cells through magnetic fluid hyperthermia (MFH). Compared with simply using FePt NPs, the spatial limitation of the layer space can further enhance the magnetocaloric effect to generate more heat energy.
The team adopted two types of liver cancer cell lines, SKHep1and HepG2, to confirm the therapeutic effect of the FePt@MMT-MIT nanosystem. After demonstrating the diagnostic efficacy of T2-weighted MRI, researchers brought FePt@MMT-MIT into mice and evaluated the treatment results.
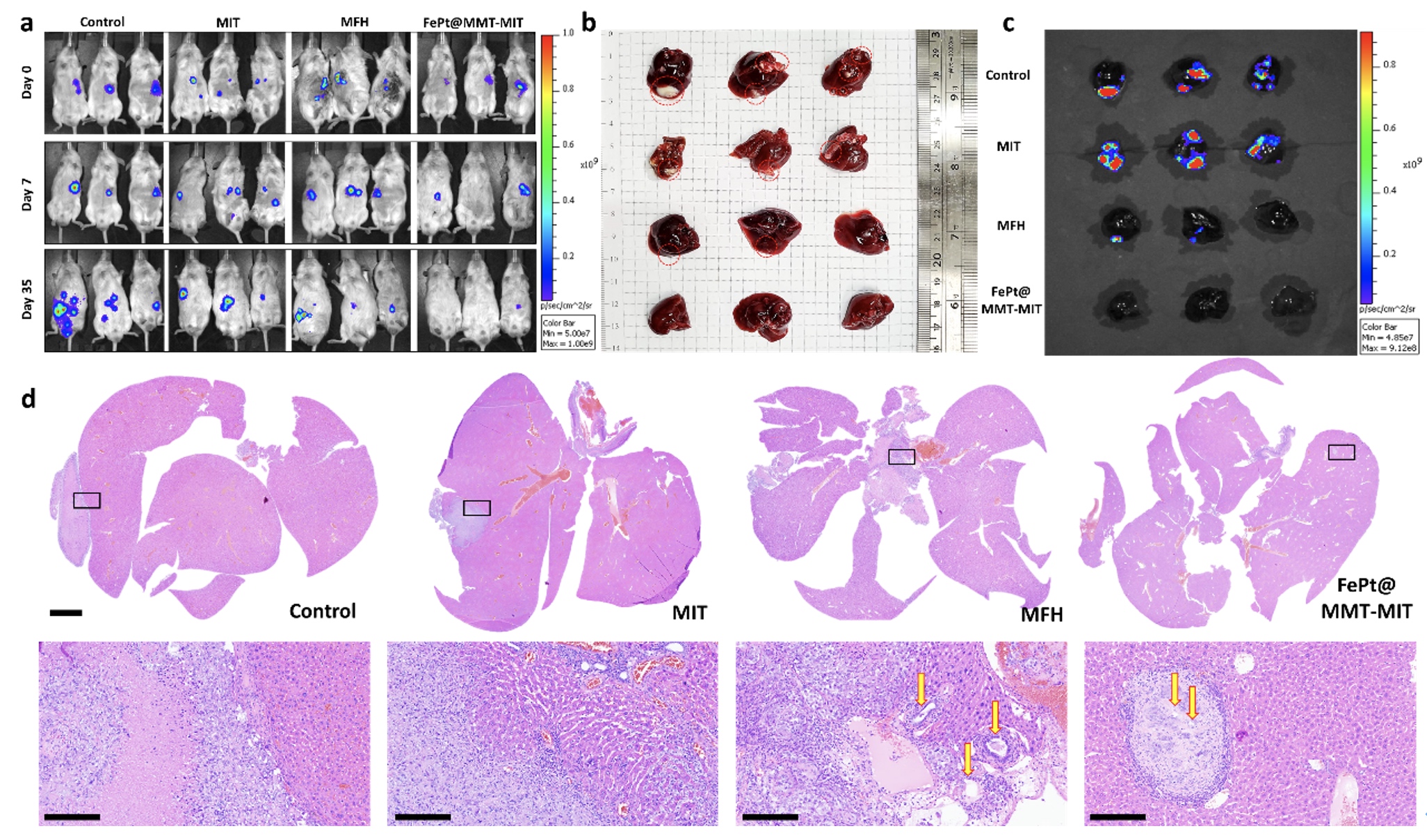
Since the orthotopic liver tumor cannot be directly observed, they used the In Vivo Imaging System (IVIS) to evaluate the effect of tumor treatment.
All the experimental groups were divided into four groups: the control group, the MIT treatment group (drug only), the MFH treatment group (FePt@MMT with external magnetic treatment but without MIT), and the MFH + MIT group (FePt@MMT under external magnetic therapy).
An excellent therapy via MFH and MIT was demonstrated with a reduction in tumor size and weight by an almost sevenfold decrease can be evaluated under IVIS investigation in Figures c and d. The MIT+MFH group is more effective in suppressing tumors than single-drug therapy (MIT and MFH).
Furthermore, the liver tumor tissues were image through the H&E staining. It is noteworthy that a clear cavity structure can be observed in the MFH group, which indicates tumor cells may cause necrosis by the heating effect and may be used as an alternative treatment for cancer and make it easier for chemotherapy-related drugs to destroy the remaining cancer cells.
Due to the inefficient suppression of tumor tissue with a single treatment, cocktail therapy has become the current standard for cancer treatment. “Our team has aimed to develop nanomaterials to provide multiple functions for a variety of illnesses,” said Dr. Ming-Hsien Chan and Chih-Ning Lu, the paper’s lead authors. Functionalized FePt@MMT-MIT could be integrated into a versatile drug delivery system by combining MRI, chemotherapeutic drugs, and magnetic guide targeting. It’s clear that there is the potential to use this in many different ways to treat cancer.
The research paper can be read online at: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-021-01052-7