CLEC18A is a member of C-type lectin family which is known to have influence over innate and adaptive antimicrobial immune responses, and its unknown biological mechanisms is to be unveiled.
In the journal Communications Biology, Professor Shi-Liang Hsieh’s team published an article and gave the insight of how CLEC18A may associate with TLR3 to enhance the immune responses to H5N1 influenza A virus (IAV) infection. Moreover, they pinpointed a single amino acid change (S339→R339) in CTLD domain of CLEC18A could have profound effect in signal transduction to increase IFN production and thus boosted anti-viral immunity two folds.
While human CLEC18A is ubiquitously expressed and highest expression levels were noted in immune cells and liver, mouse CLEC18A is only expressed in brain, kidney, and heart. On top of that, human C-type lectin 18 contains three loci, namely CLEC18A, CLEC18B, and CLEC18C, yet, there is only CLEC18A found in mice. This became a clue to the Hsieh’s team.
According to Dr. Hsieh, genetic drift between human and mouse come from polymorphism modified by natural selection. Polymorphism refers to the presence of more than two amino acids in a specific position of the gene sequence. Such differences may lead to the differences among species. Simply put, in their research work, such a subtle change may explain why same medication at the same dose can work well for some people but not others.
Hsieh’s team discovered that, the three human CLEC18A/B/C polypeptides are almost identical except polymorphic amino acid residues located in CTLD and SCP domains. In this study, they wanted to verify if CLEC18 is involved in endosomal immune responses, and, how the functions of polymorphic amino acid S339 versus R339 differ in CTLD domain of CLEC18A.
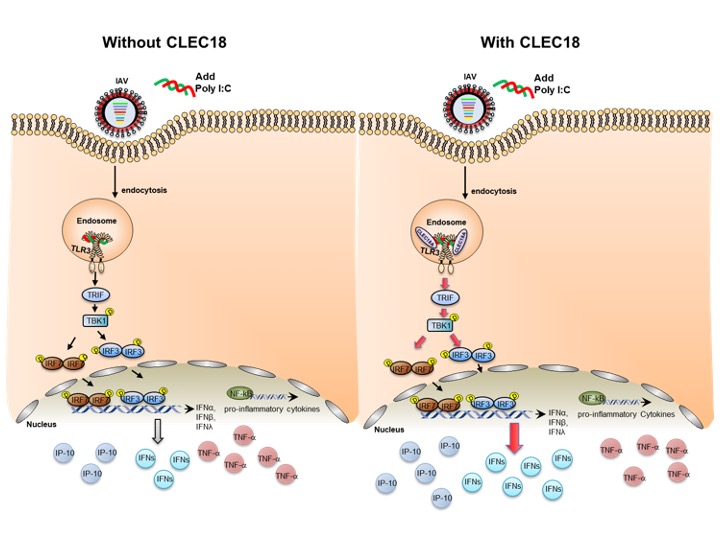
Under the fluorescence microscopic, CLEC18A and TLR3 can be tracked together in the endosome, therefore, CLEC18A may contribute to the immune responses of TLR3-mediated signaling.
“We were trying to find out the relationship between CLEC18A and TRL3,” said first author Ya-lang Huang, a postdoctoral fellow at Dr. Hsieh’s lab. “And the study lays the groundwork for understanding how they take part in immunity system.”
To recapitulate human body conditions, they introduce the model of transgenic mice. It took them over one year and they went through six to eight generations of genetically modified mice to come up with the animal model.
Collaborated with Professor Yang, An-Suei’s lab, they created an exclusive CLEC18A antibody to verify the expression level of CLEC18A in their transgenic mice.
Next, it is also necessary to prepare appropriate virus strains. The research team cooperated with the University of Hong Kong to obtain the H5N1 virus strain and transform it into less toxic synonymous, which turned out be the first H5N1 virus strain that can be carried out at P2 lab.
Comparing the physiological differences of transgenic mice and wild type mice infected with H5N1, they could find out the immune pathway regulated by CLEC18A.
 |
| The immune pathway regulated by CLEC18A |
Result showed, after exposed to the H5N1 virus, the survival rate of regular mice is 15% while the CLEC18A transgenic mice is increased to 26%. Moreover, if the No. 339 amino acid in the CTLD domain of CLEC18A is changed from S type to R type, the survival rate will jump to 53%.
Dr. Huang noted, “The improved survival rate comes from the positive immune response brought by CLEC18A.
Their study concluded that endosomal TLR3 is regarded as a sentinel to RNA viruses, and activation of TLR3 leads to IFN production. Compared to TLR3 alone, binding affinity to viruses is greatly enhanced in TLR3-CLEC18A and TLR3-CLEC18A(S339R) complexes. CLEC18A binds viruses and acts as TLR3 co-receptor to trigger the production IFNs, but not the proinflammatory cytokines when confronting H5N1 IAV infection. Compared to the wild type mice, CLEC18A and ROSA-CLEC18A(S339R) mice showed higher amounts of interferons and are more resistant to H5N1 IAV infection.
“This research not only reveals the contribution of CLEC18A to innate immunity, but also demonstrates the importance of polymorphism,” Hsieh commented. “It is interesting to note that a single amino acid change [S339→R339] in CTLD domain has such a potent effect to enhance type I and type III IFN productions and anti-viral immunity.” He also expressed interests in more studies to understand how genetic polymorphism cause individual difference. Their next plan to dig into the SCP domain in CLEC18A which is believed to be related to Fatty acid metabolism and may be a key factor of obesity!
The paper titled “Endosomal TLR3 co-receptor CLEC18A enhances host immune response to viral infection” can be read online at: https://doi.org/10.1038/s42003-021-01745-7