Pancreatic cancer is known to be vicious, pancreatic ductal adenocarcinoma (PDAC) is the type that ac-counts for 95% of pancreatic cancer and so far it is still incurable. An added fact makes PDAC doomed lies in its tendency to have metastasis, meaning cancer cells have already spread to other organs, when di-agnosed.
Obstacles physicians face in treating pancreatic cancers are quite like firefighters facing wildfires. When small fires are erupting from all directions, it would be really hard to put out the fires. Likewise, it is aw-fully hard if the cancer cells migrate to other parts of patients while the physicians are trying to treat. Therefore, blocking the cancer cells’ way out would be equally important in finding solutions to treat PDAC.
IL-17RB (Interleukin-17 receptor B) was identified as a major player in PDAC metastasis by Professor Wen-Hwa Lee’s team back in 2015. Tests had shown a direct correlation between the degree of PDAC migration and the amount of IL-17RB molecules on cancer cells. Inhibiting IL-17RB seemed a good idea, because it showed less IL-17RB meant less migration.
However, as the team dug into the topic further, they realized it was a catch situation because IL-17RB has another crucial function in the immune system. It appeared in animal tests that by blocking IL-17RB, weakened immunity led to other problems.
There is a whole family of the IL-17 receptor molecules, namely IL-17RA, IL-17R, IL-17RC, IL-17RD, and IL-17RE. Receptors are produced by cells and often show up on cell surfaces with specific missions. Every receptor has a unique partner molecule also produced from our cells, they are called ligands. Lig-ands are meant to interact with the receptor, and once they click, specific biological actions would take place. Therefore, it can be imagined as the lock and key pairs designed to perform specific tasks.
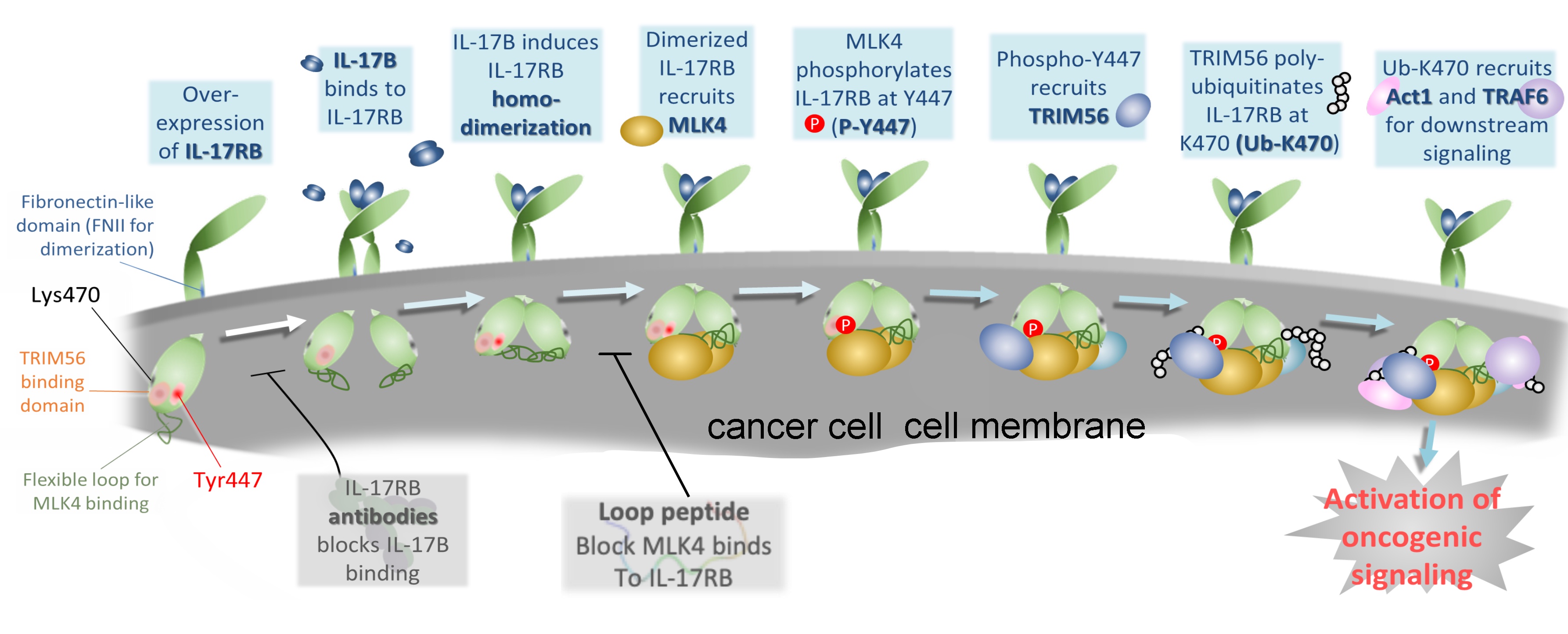 |
| Binding of IL-17B specifically induces IL-17RB homodimerization, leading to the recruitment of MLK4 and subsequent phosphorylation of IL-17RB at Y447. P-Y447 is recognized and bound by TRIM56 for K63-linked polyubiquitination at K470 of IL-17RB (Ub-K470), which initiates downstream oncogenic signaling. |
In the IL17-RB study, the team realized the true story behind the receptor/ligand interaction is much more complicated. It turned out, on the living cells, there are abundant IL-17 family receptors, and they tend to pair up to form dimer structures. Depending on the combination, the receptors form different locks for different downstream effects. For example, for the immune system to kick in, it requires the IL-17RB and IL-17RA to form a heterodimer, and this heterodimer requires a IL-17E ligand to turn it on.
For the pancreatic cancer cells, since they tend to overly express IL-17RB receptors, there are much more chances for two exactly same IL-17RBs to form a homodimer structure. And this homodimer needs a IL-17B ligand to turn on.
In the study, the first author, Dr. Heng-Hsiung Wu demonstrates that the IL-17RB homodimer will recruit MLK4, a dual kinase, then, upon meeting the key IL-17B ligand, phosphorylation takes place. The phos-phorylated IL-17RB at Y447 is known to be found abundant in the cells of pancreatic cancer cases with poor prognosis.
As a result, they traced the complete scenario to be: Homodimer IL-17RB recruits MLK4, to phosphory-late IL-17RB’s Y447, which further recruits TRIM56, TRIM56 adds K63-linked ubiquitin chains to lysine 470 of IL-17RB, which further assembles Act1 and other factors to propagate downstream oncogenic signaling.
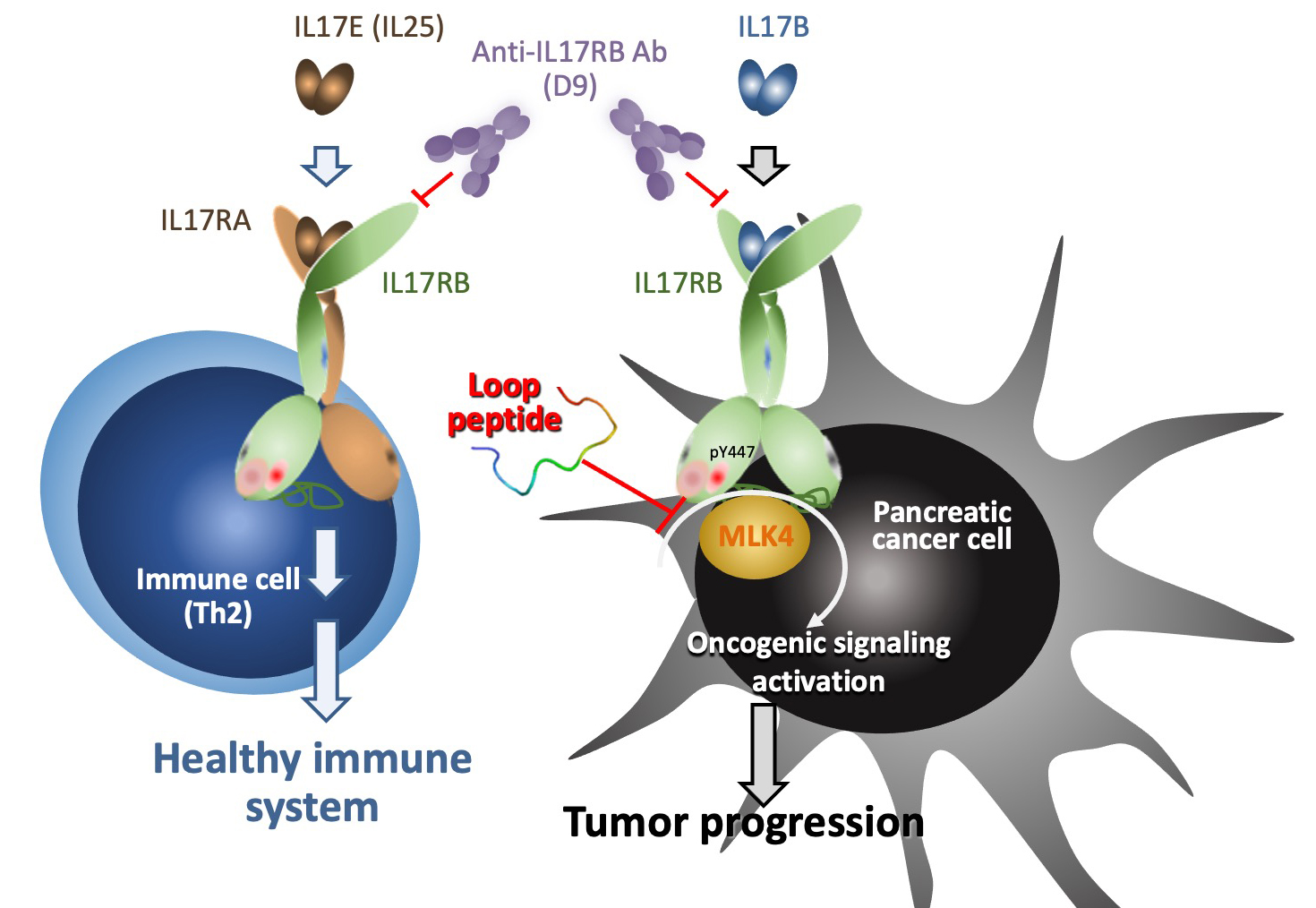 |
| In turn, interruption of the interaction between IL-17RB and MLK4 by loop peptide (TAT-IL-17RB403-416) can specifically inhibit activation of IL-17B/IL-17RB oncogenic signaling to suppress pancreatic tumor growth and metastasis. |
Not only uncovering the whole cause and effects story, the team also made a specific peptide consisting of amino acids 403-416 of IL-17RB blocks MLK4 binding, leading to loss of Y447 phosphorylation and K470 ubiquitination. This specifically designed peptide showed promising results in inhibiting tumor-igenesis and metastasis and prolonging the lifespan of mice bearing pancreatic tumors.
“The results not only establish a clear pathway of how proximal signaling of IL-17RB occurs, but also provides insight into how this pathway is clinically significant in many pancreatic cancers.”, commented Dr. Chun-Mei Hu.
Team members are from Academia Sinica (Dr. Wen-Hwa Lee, Dr. Chun-Mei Hu and Dr. Chia-Ning Shen), CMU (Dr. Heng-Hsiung Wu) and NTU physicians (Dr. Yu-Wen Tien, Dr. Yu-Ting Chang, Dr. Ming-Chu Chang and Dr. Yung-Ming Jeng.
The team has published the result in the journal Science Translational Medicine. The paper titled “Eluci-dating the Proximal Mechanistic Key Steps of IL-17 Recetor B Oncogenic Signaling for Targeted Therapy of Pancreatic Cancer” can be read online at: https://stm.sciencemag.org/content/13/583/eabc2823