A team led by Dr. Li-Jung Juan of Genomics Research Center and Prof. Yi Zhang of Harvard Medical School reported an unexpected function for Naa10p, which is primarily known to acetylate nascent peptides from ribosomes, in maintaining global DNA methylation and marking the imprinted allele for genomic imprinting maintenance. These results, published in Molecular Cell on Sep 21, also suggest that defects in DNA methylation and genomic imprinting may contribute to Naa10p-associated Ogden syndrome.
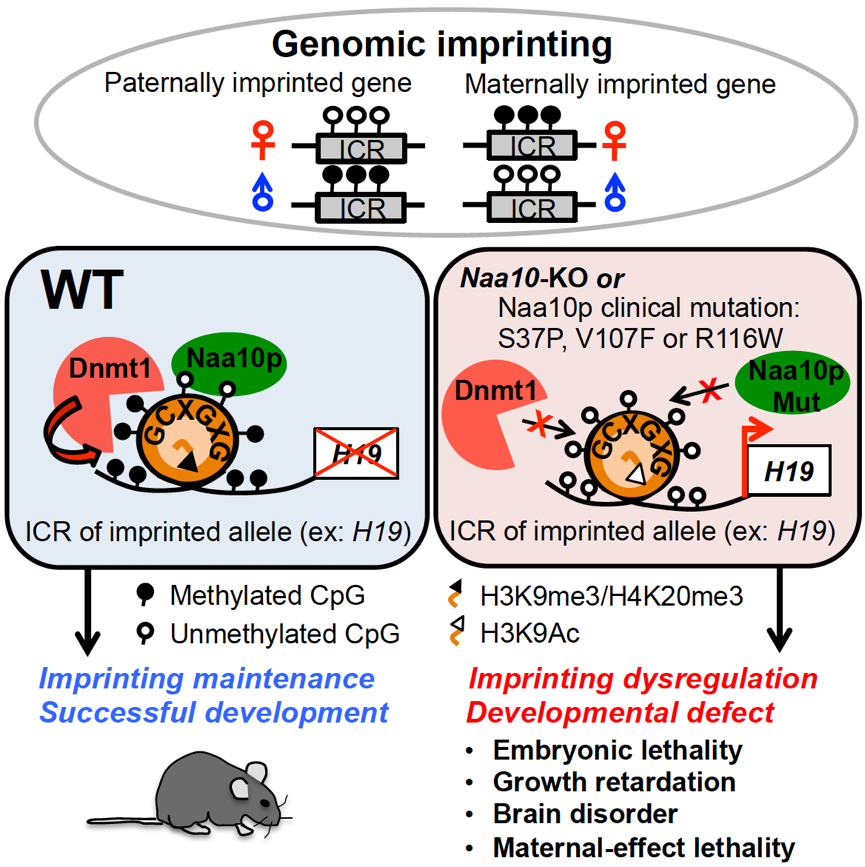
Genomic imprinting, a parental allele-specific gene expression phenomenon, is caused by allelic DNA methylation of imprinting control regions (ICRs) [also known as differentially methylated regions (DMRs)] and plays an important role in development as defects in genomic imprinting often result in developmental defects. Imprinting is established de novo during spermatogenesis and oogenesis by members of the DNA methyltransferase family Dnmt3a, 3b and 3l, maintained by Dnmt1 in pre-implantation embryos, and demethylated by ten eleven translocation 1 dioxygenase in primordial germ cells so that gamete-specific DNA methylation can be reestablished. To date, only a few proteins have been reported to be important for maintaining genomic imprinting during global DNA demethylation in the zygotes. A full account of the factors involved in imprinting maintenance is still lacking.
Mammalian N-α-acetyltransferase 10 protein (Naa10p), encoded by the X-linked gene Naa10, belongs to the highly conserved acetyltransferase family which co-translationally acetylates the N-terminal α-amino group of more than 80% of human proteins. Despite the prevalence, the physiological functions of N--acetylation and these N--acetyltransferases are largely unknown. In 2010, our laboratory demonstrated that human Naa10p contributed to tumorigenesis by facilitating Dnmt1-mediated tumor suppressor gene silencing. Importantly, starting from 2011, human Naa10p mutations have been found to cause severe developmental delays including perinatal death and intellectual disability. These observations make it very interesting to study the physiological function of Naa10p.
The current study reports that Naa10-null mice display partial embryonic lethality, growth retardation, brain disorders and maternal-effect lethality, phenotypes commonly observed in defective genomic imprinting. Genome-wide analyses further revealed global DNA hypomethylation and enriched dysregulation of imprinted genes in Naa10p-knockout embryos and embryonic stem cells. Mechanistically, Naa10p maintained DNA methyltransferase 1 (Dnmt1) activity by facilitating Dnmt1 binding to DNA substrates including the ICRs of the imprinted allele during S phase. Moreover, the lethal Ogden syndrome-associated mutation of human Naa10p disrupted its binding to ICR of H19 and Dnmt1 recruitment. Our study thus links Naa10p mutation-associated Ogden syndrome to defective DNA methylation and genomic imprinting.
Most experiments were carried out by the first author Dr. Chen-Cheng Lee, an Academia Sinica Distinguished Postdoc Fellow at Dr. Li-Jung Juan’s laboratory, with critical help from two co-first authors Shih-Huan Peng from Institute of Molecular Medicine, National Taiwan University and Dr. Li Shen from Dr. Yi Zhang’s laboratory. International collaborators also include Prof. Xiaodong Cheng from MD Anderson Cancer Center and Prof. Guo-Liang Xu of Chinese Academy of Sciences at Shanghai Institutes for Biological Sciences.
Contact: Dr. Li-Jung Juan This email address is being protected from spambots. You need JavaScript enabled to view it.,edu.tw
Link to paper: http://www.cell.com/molecular-cell/fulltext/S1097-2765(17)30623-8
 |
|
Naa10p KO causes defects in genomic imprinting and embryonic development. Naa10p maintains Dnmt1 activity by facilitating Dnmt1 binding to DNA substrate. Naa10p selectively binds to ICRs of the imprinted allele via non-methylated GCXGXG. Ogden syndrome-causing Naa10p mutation disrupts ICR binding of Naa10p and Dnmt1. |