To save lives, man develops antibiotics to kill bad bugs, which otherwise foster super bugs to emerge. To fight back the invincible super bugs by developing more effective antibiotics sounds paradoxical, but it is a fate, a never ending story.
The research team led by Dr. Tsung-Lin Li at the Genomics Research Center, Academia Sinica, has made a breakthrough that could ease the bacterial havoc. Dr. Li and his colleagues have long been dedicated to new drug discovery in particular for serious bacterial infectious diseases. As a matter of fact, bacteria are acquiring antibiotic resistance at a rate that outpaces new drug development.
A solid step made to break the curse done by the Li’s team was revealed in the latest advanced online publication of Nature Chemical Biology (April 10th). And, a follow-up report by The Scientist magazine was posted right afterwards.
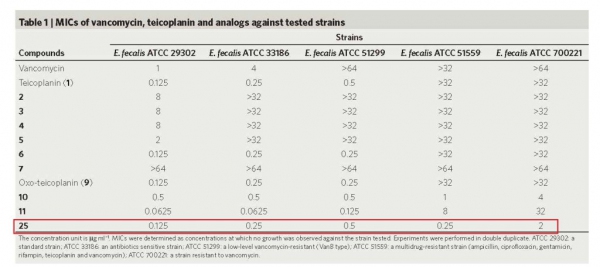
The team has created a small library of new compounds from an enzyme-added solution. In the tests against collected drug-resistant bacterial strains, these compounds showed superb in vitro bug-killing effect as 100-fold as the so-called drugs of last resort vancomycin. In the tests of mice infected with the drug-resistant strains, the given drugs also performed more than satisfactory in in vivo efficacy.
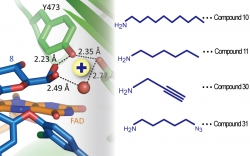
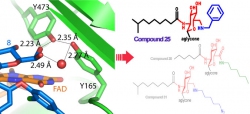
Glycopeptide antibiotic A40926 is a natural product similar to vancomycin. The research team indicated that Dbv29 is a hexose oxidase involved in the maturation of A40926, for which the enzyme can oxidize the n-acyl glucosamine moiety of the antibiotic into n-acyl glucuronic acid. A pair of active site tyrosine residues (Y165/Y473) and cofactor FAD (flavin adenine dinucleotide) were determined by X-ray crystallographic and biochemical experiments to be the key factors in the catalysis.
Mr. Yu-Chen Liu, who is a doctoral student of Chemical Biology and Molecular Biophysics (CBMB) program, Taiwan International Graduate Program (TIGP), Academia Sinica, is the first author of this paper. Liu said that Dbv29 is a hexose oxidase that oxidizes primary alcohol into carboxylic acid via an aldehyde intermediate. To their surprise, that the un-isolatable diol intermediate appeared in the teicoplanin-bound protein crystal structure enabled them to brood out aldehyde-amine-chemistry coupled enzyme reactions. By adding various building blocks, they successfully tweaked the tecoplanin compound into new compounds.
Encouragingly, they found teicoplanin tailored with given building blocks have broad biological activities, in which some show superior bactericidal effect against vancomycin-resistant enterococci (VRE) to vancomycin and teicoplanin.
The full-text of the study entitled “Interception of teicoplanin oxidation intermediates yields new antimicrobial scaffolds” is available at the Nature Chemical Biology website. This work was supported by the Genomics Research Center of Academia Sinica, National Science Council, and National Synchrotron Radiation Research Center.
A-IMBN RESEARCH Highlights:
" Building a better germ-killer" May 18, 2011