Pseudomonas aeruginosa is one of the most common pathogens for nosocomial infections worldwide. It is commonly found in chronically ill, immunocompromised, and aging peoples. Patients with open wounds, respiratory intubation or drainage tubes are also highly susceptible for P. aeruginosa infection. Even though Pseudomonas aeruginosa are sensitive to antibiotics in early stage, drug resistance strains emerge quickly after treatment, and the course of disease progress rapidly form local to systemic infection.
The research team led by Dr. Shie-Liang Edmond Hsieh, distinguished professor in the Genomic Research Center of Academia Sinica, published an article in JCI Insights recently to reveal a novel mechanism of P. aeruginosa-induced lung injury and develop a novel approach to increase treatment efficacy as well as attenuate tissue damage. This discovery bring a novel strategy and direction to treat bacterial infections.
After the invasion of P. aeruginosa, neutrophils and macrophages are the most important innate immune cells to limit bacterial spreading. It is well known that the innate immune receptor TLR4 is critical in controlling the invasion of P. aeruginosa, but it was not understood why pathogenic P. aeruginosa, even though antibiotics can limit its growth and invasion, still causes severe and intense immune responses leading to damage of lungs and airways.
When encountering large-scale bacterial invasion, the over-stimulated neutrophils struggle to expand their chromosomes first, rupture the nucleus, and then release a large number of sticky filamentous chromosomes, wrapping the pathogens in clusters and engulfing them. The sticky structure released from the overactivated neutrophils are known as "Neutrophil Extracellular Traps (NETs)", and such a process is called NETosis. However, NETs are "double-edged swords", as overproduction of NETs causes excessive inflammation in the body. Although NETs have antibacterial effects on some less virulent bacteria (such as Listeria monocytogenes and Staphylococcus aureus), Dr. Hsieh's team found that NETs do not help host against P. aeruginosa invasion. In contrast, NETs contribute to P. aeruginosa-induced lung inflammation and fibrosis. Thus, it is crucial to identify the key receptor responsible for P. aeruginosa-induced NET formation and lung damage.
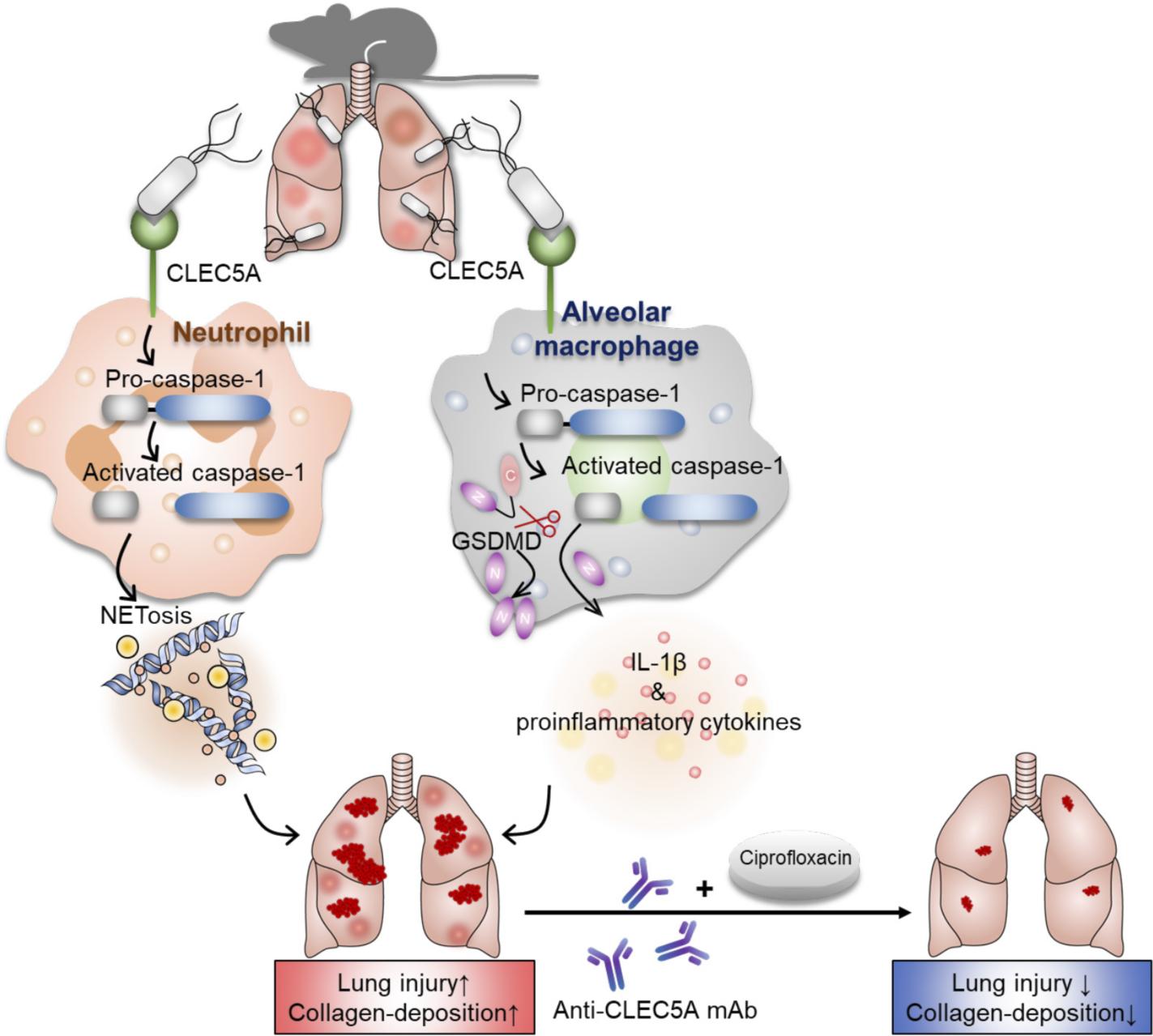 |
| P. aeruginosa induce NETosis and inflammatory cytokines to cause tissue damge via CLEC5A. |
As early as 2017, Dr. Hsieh's research team has published an article in the journal "Nature Communications" that CLEC5A (C-Lectin-5A, C-type lectin 5A) is responsible for L. monocytogenes (a Gram-positive bacterium)-induced NET formation, and plays protective role to eradicate L. monocytogenes invasion(Research news: CLEC5A proved to be a key player that triggers NET formation (NETosis) after bacterial infections). However, Dr. Peishan Song, the first author of this JCI Insights paper, found that P. aeruginosa engages CLEC5A to trigger a stronger inflammatory response via activating caspase-1, which further triggers downstream signaling to induce robust NETosis. In addition to neutrophils, activation of CLEC5A also leads to cleavage of Gasdermin D (GSDMD) and conversion of pro-IL-1β to IL-1β to initiate pro-inflammatory response in alveolar macrophages. However, the activation of CLEC5A in neutrophils and alveolar macrophages is ineffective to limit P. aeruginosa invasion, resulting to overproduction of NETs and proinflammatory cytokines to cause lung damage, acute pneumonia, and more serious consequences.
While all the mice knocked out of TLR4 all died, mice knocked out of CLEC5A maintained a survival rate of 100% after 120 hours inoculation, suggesting TLR-4 is an indispensable defense receptor, but CLEC5A is detrimental in P. aeruginosa infection
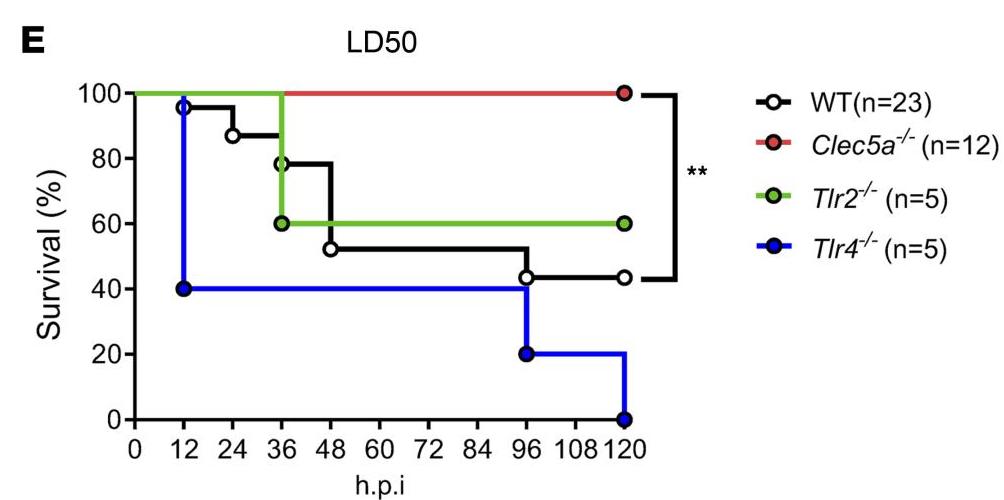 |
| Survial rate of TLR2、TLR4 and CLEC5A knocout mice. |
"If we block the CLEC5A receptor, will it calm down the inflammatory responses, increase survival rate, and attenuate tissue damage in P. aeruginosa infection?" the research team thought. Bingo! Dr. Hsieh's team validate this idea by simultaneous injection of anti-CLEC5A monoclonal antibody (anti-CLEC5A mAb) and broad-spectrum antibiotic Ciprofloxacin into experimental mice inoculated with P. aeruginosa. The results showed that mice injected with both anti-CLEC5A mAb and Ciprofloxacin had a survival rate of nearly 90%, compared with 70% of mice given antibiotics alone and less than 30% of mice given antibodies alone. Furthermore, antibiotic alone only has bactericidal efficacy but cannot reduce inflammation, thereby causes severe lung fibrosis even after recovery. Confocal microscopy imaging showed that combination of antibodies and antibiotics reduce collagen deposition in the lungs of infected mice.
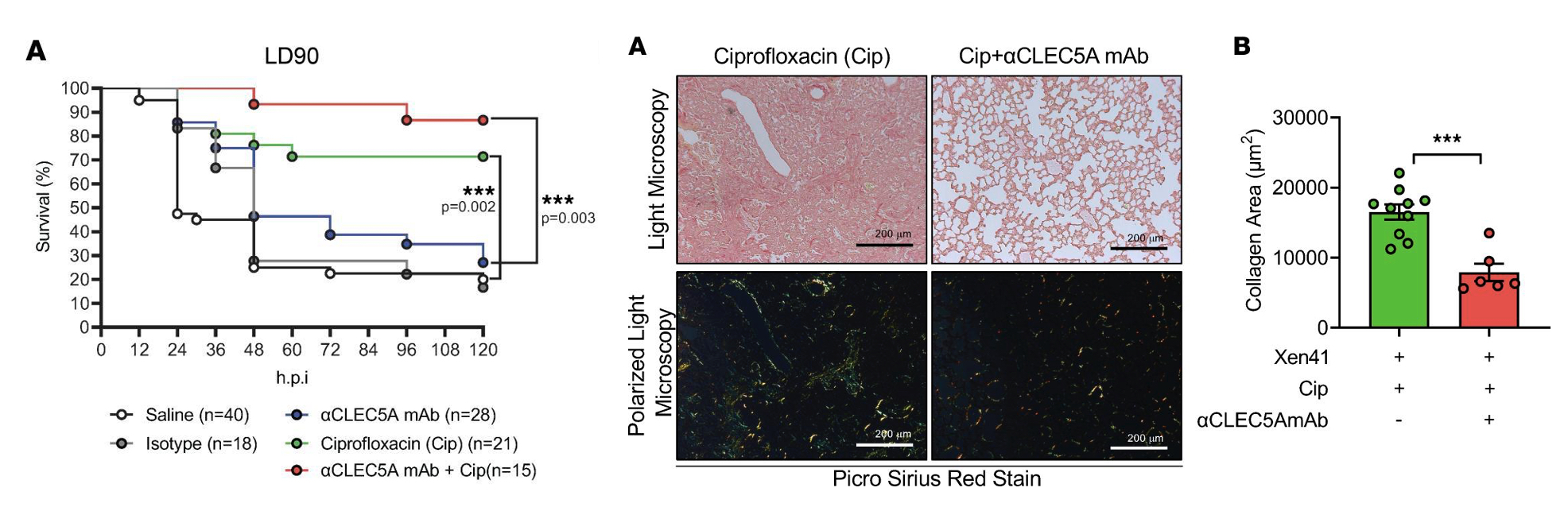 |
| Simultaenous injection of anti-CLEC5A mAb with Ciprofloxacin increases survival rate and attenuates collagen depostion in lung after P. aeruginosa infecction. |
This study once again confirmed that CLEC5A plays a pivotal role in the regulation of NETosis and pro-inflammatory cytokines during bacterial infection. The anti-CLEC5A mAb can inhibit inflammation, soothe the patient's discomfort, reduce damage to normal tissues, and accelerate the complete recovery of the body. This finding proposes combination of anti-inflammation (anti-CLEC5A mAb) and bactericidal effect (antibiotic) would be a new direction for the treatment of nosocomial infection caused by P. aeruginosa. Dr. Hsieh also point out that patients suffered from cystic fibrosis, which is caused by a defect in the CFTR gene, frequently associate with chronic persistent P. aeruginosa infection in lung because immune system is unable to clear P. aeruginosa and results in drug-resistant strains. It would be interesting to test whether anti-CLEC5A mAb will be also help to clear P. aeruginosa infection and prevent tissue damages in patients with cystic fibrosis.
This major contributor to this research work is Dr. Peishan Song, and this work is in collaboration with Cheng-Hsun Chiu, Vice President of Chang Gung Hospital. We are grateful to Professor Richard Cumming in Harvard University to provide the PAO1 strain for this work. The research paper "CLEC5A is critical in Pseudomonas aeruginosa–induced NET formation and acute lung injury" is available online: https://insight.jci.org/articles/view/156613.