A research team led by Dr. Che Ma and Dr. Wen-Hwa Lee in the Genomics Research Center reported they solved complex structure of IL-17RB with D9, and this structure provides important paratope information to guide the design of antibody humanization and affinity maturation of D9. Eventually, the affinity matured humanized antibody clone 1B12 was demonstrated significant inhibition of pancreatic tumorigenesis in an orthotopic mouse model. This research has been published in the journal "Cell Reports".
Back to 2015, Dr. Lee’ team have found that IL-17RB plays a distinct role in promoting tumor growth and metastasis upon stimulation with IL-17B. The upregulation of IL17RB leads to the worsened prognosis in pancreatic cancer patients as well as in breast tumors. (Huang, C.K., etc. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-κB-mediated antiapoptotic pathway. Oncogene. 2013; 33:2968–2977. DOI: 10.1038/onc.2013.268; HH Wu, etc., Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212(3):333-49. DOI: 10.1084/jem.20141702.)
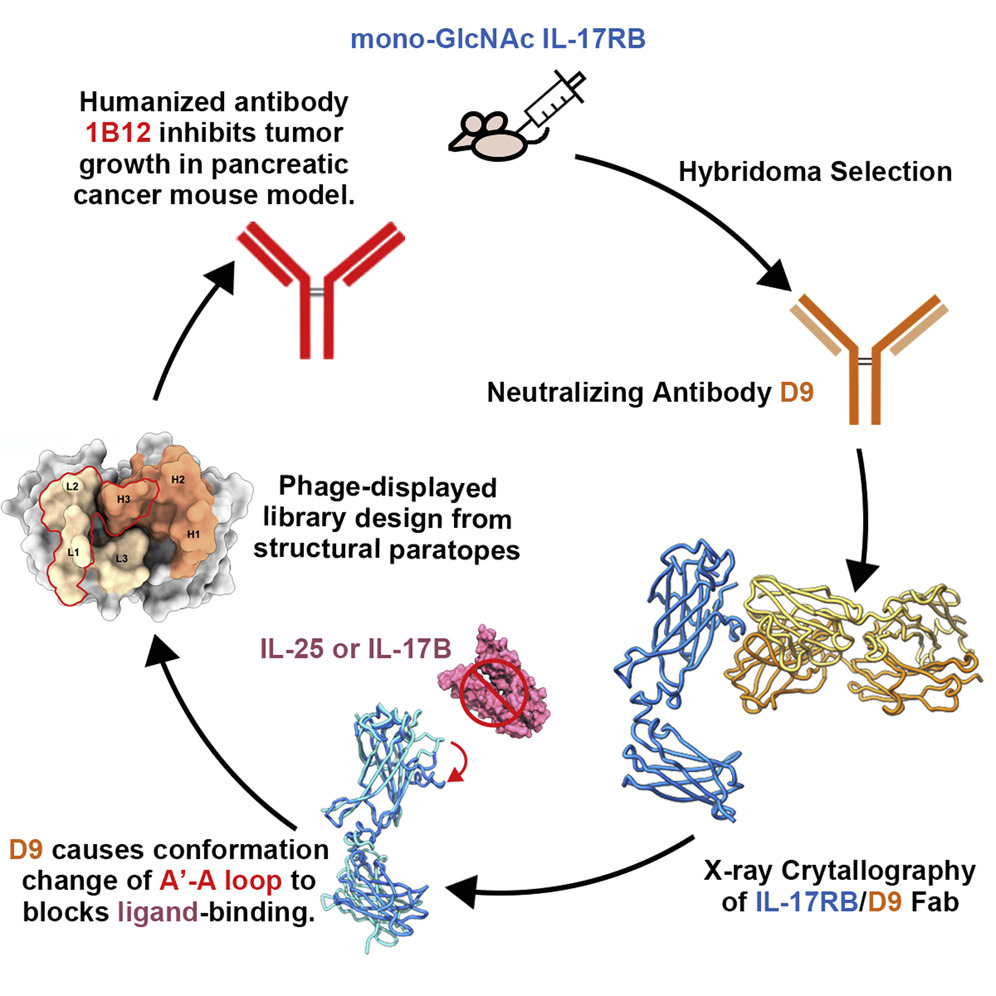
The research group of Dr. Ma and Dr. Lee reported an IL-17-RB-neutralizing monoclonal antibody D9 that could block IL-17RB/IL-17B pathways and inhibit pancreatic tumorigenesis in an orthotopic mouse model.
However, D9 is from the mouse origin and its humanization is a necessary process for therapeutic antibody development, which can reduce undesired rejection reaction.
Based on research from Dr. Ma have shown that removal of the glycan shield could elicit higher immune response, the team described the specific deglycosylation strategy to generate the neutralizing monoclonal antibody D9 from mice immunized with mono-GlcNAc decorated IL-17RB.
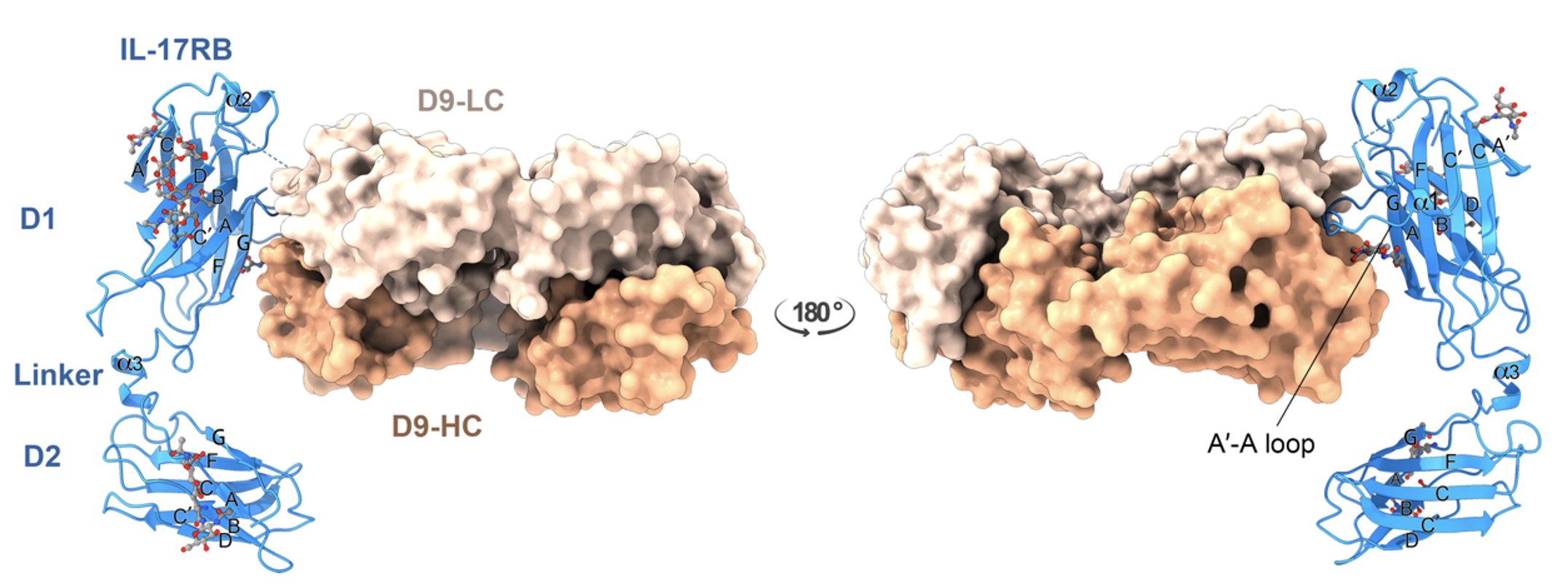
With highly potent antibodies, they further solved the X-ray crystal structure of D9 Fab domain in complex with the homologues bovine IL-17RB ectodomain. The structure shows that D9 binds to a predicted ligand-binding interface and engages with the A′-A loop of IL-17RB fibronectin III domain 1 in a unique conformational state.
 |
| X-ray crystal structure of bIL-17RB ECD with cD9 Fab |
These findings have provided key insights into the inhibitory mechanism of D9 that its interaction with the A'-A loop would cause the natural ligand binding site conformational change, prevent the ligand engagement, in consequence of blocking the downstream signaling cascades of IL-17B/IL-17RB in cancer proagression.
The study’s lead author, Wen-Hsin Lee, a PhD student at the Dr. Ma’s Lab, noted: “Combining the structural and functional information of the IL-17RB/D9 interface has provided us with an invaluable guidance for generating therapeutically useful anti hIL-17RB antibodies.”
The first step of humanization is replacing non-human antibody frameworks with human ones. She engrafted six CDRs into the framework of the commercialized anti-HER2 (human epidermal growth factor receptor 2) antibody, Trastuzumab, with the human IgG1 constant fragment (Fc).
To increase the binding affinity of the humanized D9 and retain the high neutralization potency as mouse D9, Lee then designed a phage-displayed library from structure paratopes to create hD9 variants only in the regions of HCDR3, LCDR1, and LCDR2 to minimize the influence on the epitope-paratope interaction and structural integrity.
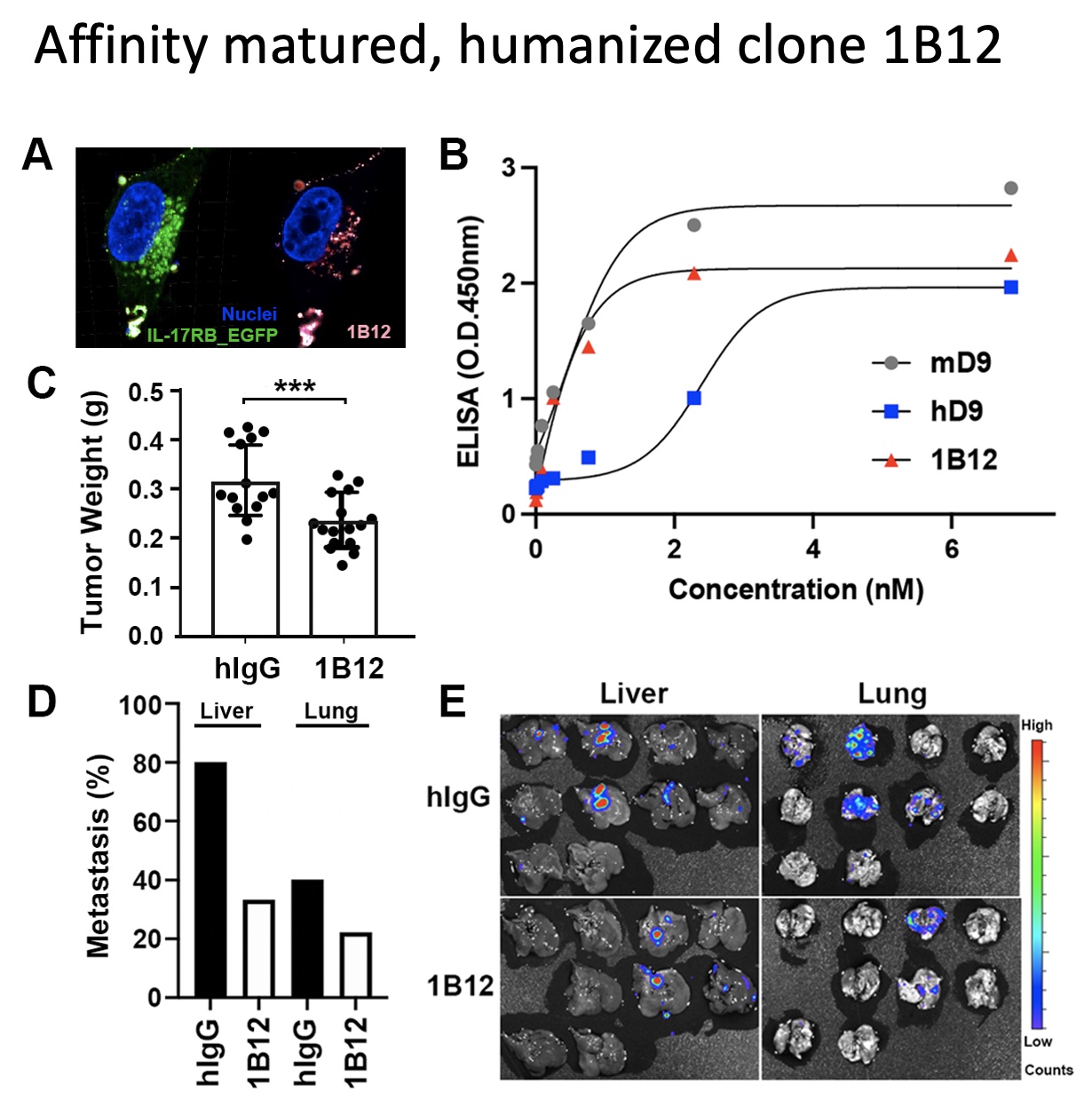
Finally, an affinity matured humanized antibody clone, 1B12, was selected for its high specificity and increased binding avidity to IL-17RB, which is five times higher than the parent clone mouse D9.
 |
| The design of antibody humanization and affinity maturation of D9 |
In the mouse orthotopic model, injections of 1B12 (5mg/kg per week) can significantly decrease the tumor growth and metastasis comparing to the control human IgG, as demonstrated by the tumor weight of the 1B12 group being 1.34x smaller than the control. These results suggest that the affinity maturation of hD9 such as the resulting humanized 1B12 antibody can efficiently recognize hIL-17RB with similar affinity and inhibit the proliferation of pancreatic cancer cells in vivo.
Although it is still in the very preliminary stage for the development of the IL-17RB antagonist antibody, these results have undoubtedly become a key step forward and suggested the potential value of D9 and 1B12 in the therapeutic application for cancer treatments in the future.
The paper titled “Structural basis of interleukin-17B receptor in complex with a neutralizing antibody for guiding humanization and affinity maturation” can be read online at: https://www.cell.com/cell-reports/fulltext/S2211-1247(22)01411-5