Natural products are a significant source of structurally diverse bioactive compounds for pharmaceutical, cosmetic, and food applications. Bioactive compounds often carry desired biological activities, such as anti-inflammation, anticancer, analgesics, and pain killing.
The search for novel bioactive compounds from the Mother Nature will never dwindle, as we confront emerging diseases from time to time, for example, drug-resistant diseases. Deepening the knowledge of natural products chemistry alongside their biosynthetic logics is the crux in the discovery/development of new cures.
Professor Tsung-Lin Li and his team recently characterized an interesting biocatalyst named as StnK3, which elegantly controls the chirality of resulting products in the biosynthesis of streptonigrin. They fur-thered the adventure into its catalytic efficiency, selectivity and environmental friendliness, which enable them to design novel metabolites and fine chemicals. This work has been published in the field’s leading journal "ACS Catalysis".
β-Amino acids have been found broadly employed as components in many clinically important natural products. They found StnK3 is the key player mediating the chirality-inversion between (2S,3R) and (2S,3S) β-methyltryptophan.
By determining the crystal complexes, StnK3 was revealed as a Fe3+-dependent epimerase, in which the iron is held in place by a 3-His-1-Glu tetrad alongside an α-keto acid bidentate from the substrate to form a six-coordinate octahedral complex. This geometry props up a novel Lewis acid-promoted enolization/coordination-switching mechanism for the epimerization to take place.
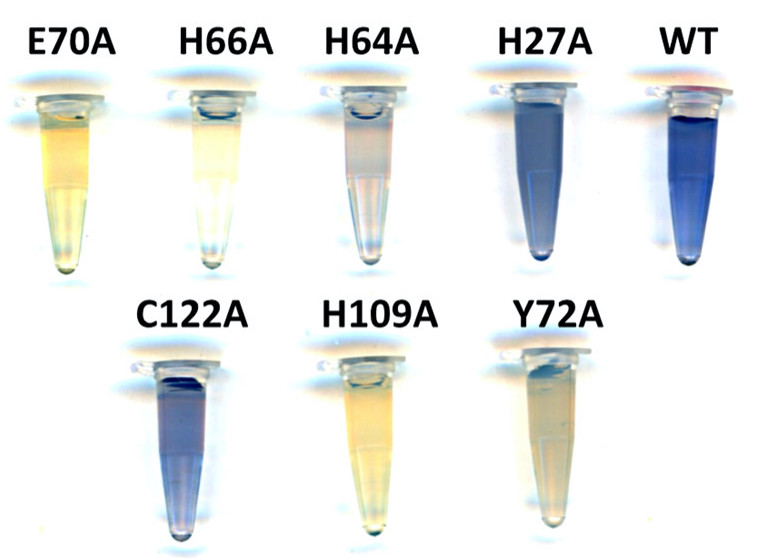 |
| The color variation amid WT and its mutants suggests that each ligand per se coordinates with the metal ion in varied magnitude. |
In essence, StnK3 evolves an octahedral/hexahedral coordination-switching device that recognizes (3R)-β-MeInPy on the one hand, triggers the Lewis acid-mediated enolization on the other hand, resulting in Glu70 freed from the coordination and allowing inverse reprotonation at the opposite side of the intermediate to yield (3S)-β-MeInPy, thus revealing an unprecedented “hidden” two-base mechanism.
“To the best of our knowledge, that a given proton in a ligand internally returns back to itself in an enzyme-mediated epimerization reaction following the two-base mechanism is likely the first of its kind.” said Dr. Li.
Additionally, the study also showed that StnK3 possesses an unexpected proofreading prowess, whereby the unmatured upstream substrate indolepyruvate is cleaved to indole aldehyde and glycolic acid via a retro-aldol reaction, thereby avoiding deadweight loss but assuring reaction toward the mature final product.
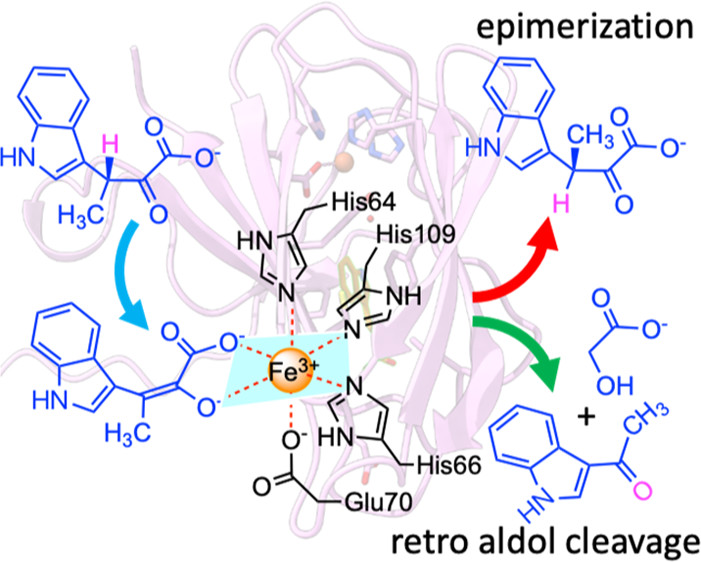
Mutant H27A converts the epimerase to a full-duty retro-aldolase, which remarkably breaks the C−C bond of unfavorable β-MeInPy to 3-acetylindole and glycolic acid at catalytic scope.
This finding opens up a new avenue to synthetic chemistry for chemically-difficult-to-synthesize compounds in a greener way, for example, to cut down energy consumption or carbon footprint.
To this end, the discovery of new reactions and elucidation of committed mechanisms not only help crack how the regioselectivity is implemented but also lay a ground to aid design of new biocatalysts for the synthesis of industrially or medicinally useful chemicals.
The paper titled “Structural and Mechanistic Bases for StnK3 and Its Mutant-Mediated Lewis-Acid-Dependent Epimerization and Retro-Aldol Reactions“ can be found online at: https://pubs.acs.org/doi/10.1021/acscatal.1c04790