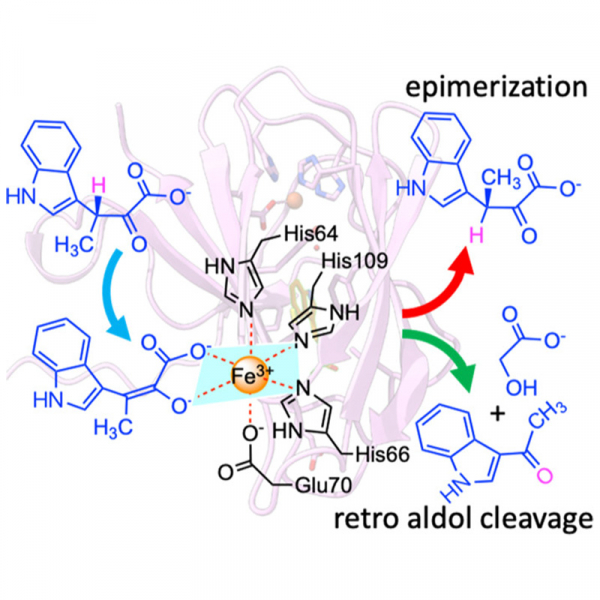
β-Methyl amino acids are important building units contributing to diversification of natural products, of which stereoselectivity and reaction fidelity are critical issues but far from conclusive. StnK3 is an enzyme mediating the chirality-inversion between (2S,3R) and (2S,3S) β-methyltryptophan. Here, we report that StnK3 is a novel Fe3+-dependent epimerase, in which the iron is structurally held by a 3-His-1-Glu tetrad alongside an α-keto acid bidentate from the substrate to form a six-coordinate octahedral complex. In addition to recognizing β-methylindolepyruvate (β-MeInPy), this given setting increases β-C acidity of the substrate facilitating its enolization; subsequent epimerization is implemented by inverse protonation utilizing a hidden coordination-switching device through an unprecedented two-base internal return mechanism. Beyond that, StnK3 exhibits an additional proofreading activity, where the immature substrate indolepyruvate (InPy) is cleaved to indole aldehyde by a retro-aldol reaction, thereby avoiding futile cycling but assuring reaction fidelity. Based on this, mutant H27A converts the epimerase remarkably to a full-duty retro-aldolase that breaks the C–C bond of β-MeInPy instead of InPy to 3-acetylindole at catalytic scope. The discovery of new reactions and elucidation of committed mechanisms not only help crack how the regio- and enantioselectivity is implemented but also lay a foundation to aid design of new biocatalysts/reactions for the synthesis of industrially or medicinally useful chemicals.
Language
週四, 20 一月 2022 10:09