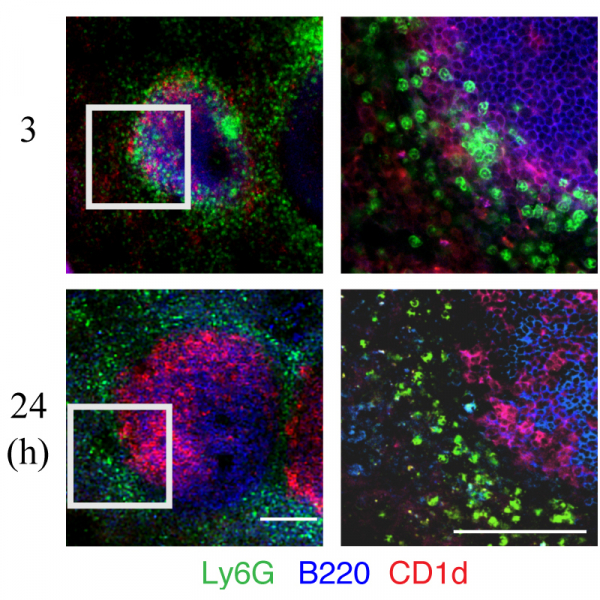
In addition to regulating immune responses by producing antibodies that confer humoral immunity, B cells can also affect these responses by producing cytokines. How B cells participate in the clearance of pathogenic infections via functions other than the production of pathogen-specific antibodies is still largely unknown. Marginal zone (MZ) B cells can quickly respond to bacterial invasion by providing the initial round of antibodies. After a bloodborne bacterial infection, neutrophils promptly migrate to the MZ. However, the mechanisms regulating neutrophil accumulation in the MZ during the initial phase of infection also remain obscure. Here, we found that MZ B cell-deficient mice are more susceptible to systemic Staphylococcus aureus (S. aureus) infection compared with wildtype mice. The expression levels of interleukin (IL)-6 and CXCL1/CXCL2 in MZ B cells increased significantly in mice at 3–4 h after infection with S. aureus, then decreased at 24 h post-infection. After systemic S. aureus infection, splenic neutrophils express increased CXCR2 levels. Our results from confocal microscopy imaging of thick-section staining demonstrate that neutrophils in wildtype mice form cell clusters and are in close contact with MZ B cells at 3 h post-infection. This neutrophil cluster formation shortly after infection was diminished in both MZ B cell-deficient mice and IL-6-deficient mice. Blocking the action of CXCL1/CXCL2 by injecting anti-CXCL1 and anti-CXCL2 antibodies 1 h before S. aureus infection significantly suppressed the recruitment of neutrophils to the MZ at 3 h post-infection. Compared with peptidoglycan stimulation alone, peptidoglycan stimulation with neutrophil co-culture further enhanced MZ B-cell activation and differentiation. Using a Förster resonance energy transfer by fluorescence lifetime imaging (FLIM-FRET) analysis, we observed evidence of a direct interaction between neutrophils and MZ B cells after peptidoglycan stimulation. Furthermore, neutrophil depletion in mice resulted in a reduced production of S. aureus-specific immunoglobulin (Ig)M at 24 h post-infection. Together, our results demonstrate that MZ B cells regulate the rapid neutrophil swarming into the spleen during the early phase of systemic S. aureus infection. Interaction with neutrophils assists MZ B cells with their differentiation into IgM-secreting cells and contributes to the clearance of systemic bacterial infections.
Language
週一, 10 五 2021 13:22