On the fifth floor lab zone of the center, we encountered the friendly smiling faces from team members in Professor Chang’s lab. The size of the team seems relatively small, and the easy going atmosphere was a surprise to us. After all, this is the lab that just signed a relatively large amount of licensing payment with a local biotech company. It has the potential of being the birth place of a powerful new drug.
Since it is rare that a technology transfer deal is reported by multiple press media, we hoped to get some insider scoops so that we could share them with other researchers. More than willingly, Professor Chang and his team agreed to spare some time with us.

Professor Tse Wen Chang has already achieved a remarkable track record in new drug development. The anti-allergy drug, Xolair, a new drug having been approved for severe allergic asthma and shown to be effective for allergic rhinitis, food allergy, atopic dermatitis, and other allergic diseases, has brought him much recognition and financial rewards. Prof. Chang is not only the inventor of Xolair but also participated in all phases when Xolair was tested, verified, and finally approved. Xolair is the first drug of its kind conceptually and technologically, since it was conceived against the conventional wisdom and developed with the state-of-the-art antibody engineering platform.
In short, Xolair functions in a treated patient by targeting IgE, which is produced by B lymphocytes of the immune system. IgE is part of the immune system, which has been evolved to fight off parasites. However, for many people living in a modern environment with a modern lifestyle, IgE does not seem to be essential for immune defense. On the contrary, many people produce IgE toward harmless environmental antigens, causing narrowing of the airway and a large array of inflammatory reactions, which result in many types of allergic diseases.
For many years, it was unclear whether IgE was involved in the pathogenesis of asthma and some other allergic diseases. The invention of Xolair by Dr. Chang and the numerous clinical studies with Xolair in various allergic diseases have clearly established that IgE is the primary mediator of many types of allergic diseases. Indeed, if IgE is neutralized in a patient, the allergic reactions subside.
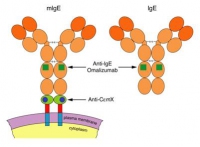
Xolair is an antibody that binds to human IgE with a unique set of binding specificities. It can neutralize essentially all freely floating IgE in the blood stream and in the interstitial space in a treated patient. In fact, there are two types of IgE. One is “secreted IgE”, freely circulating in the blood, lymph, and other body fluids. The other is “membrane-bound IgE (mIgE)”, plugged on the cell membrane of B cells. At a crucial differentiation stage, a single B cell has approximately 200,000 to 500,000 copies of mIgE on it.
While Xolair has been proven to be effective, it has its short comings. It reacts with free IgE, and therefore, patients usually require continual dosing of hundreds of milligrams of Xolair every two or four weeks. Patients with very high IgE levels in blood, who account for 15% among patients with severe allergic asthma and much higher proportions in patients with atopic dermatitis, are excluded from using Xolair in the health care system.
IgE was discovered by two teams of immunologists in 1967. After 20 years, Dr. Chang invented the therapeutic concept of anti-IgE (Xolair) in 1987. Subsequently in 1991, Dr. Chang, while pondering a way to target the root of IgE production, discovered a domain of 52 amino acid residues on mIgE. He named this previously unknown region “CεmX”, which transpires the meaning of “a constant region of unknown function on the membrane-bound chain”. Dr. Chang said that the discovery is a gift from God, because it CεmX is an extremely attractive target for therapeutically intervening with the IgE-producing pathway.
Dr. Chang joined Genomics Research Center in 2006. All his graduate students moved with him from the College of Life Science, National Tsing Hua University, including the three students who contributed to the anti-CεmX research and were co-inventors of the patent application on anti-CεmX, which is the subject of the licensing reported by the media recently.
Among the three students, Chen Jiun-Bo started learning immunology early in his training. In his sophomore year, he already took Prof. Chang’s very demanding immunology course, designed for senior students. Jiun-Bo got his Ph.D. in 2010, and has been continuing research on Anti-CεmX as a postdoctoral fellow in Dr. Chang’s lab.
Wu Pheidias is currently a student working toward his Ph.D. degree. He studied material and chemical engineering as an undergraduate. Unlike his classmates, he chose to pursue advanced biomedical training instead of landing a relatively richer-paying industrial job.
Hung Alfur Fu-Hsing is also a pre-doctoral student, whose undergraduate major was chemistry. He also followed his own inner call and chose bioscience. He recalls that Prof. Chang required him to thoroughly read a molecular biology and an immunology textbook and took courses in these subjects before accepting him to study in his lab.
In the study of Anti-CεmX, the three students each have his own interest, but, they share a common goal, which is to explore research projects that bear practical value. They also find that brain storming and knowledge sharing process is an enjoyable and crucial element in their work.
The initiative of the study was mostly from Jiu-Bo’s idea of developing a new Anti-CεmX monoclonal antibody that would have a set of properties suitable for therapeutic development. He reasoned that such an antibody must be developed with an approach taken previously in the same laboratory.
Meanwhile, Alfur was working on expressing and purifying the membrane type IgE, in an effort to provide material for undertaking X-ray crystallographic structure studies in collaboration with Dr. Alex Ma’s group. Alfur reasoned that his membrane–bound form of IgE contains CεmX in native conformation and should provide as a much improved antigen to immunize mice for preparing hybridomas secreting Anti-CεmX monoclonal antibodies.
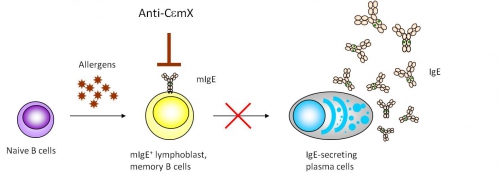
Figure 3 illustrates the rationale for the therapeutic approach that employs an Anti-CεmX monoclonal antibody. As a sensitized individual continues to be exposed to the antigen the person is sensitized to, the antigen-specific naïve B cells in the person interact with the antigens and differentiate via several steps to become plasma cells that secrete IgE specific to the antigens. In this B cell differentiation pathway, the B cells go through the B lymphoblast stage and some of those B cells become memory B cells, which potentiate IgE response in repeated antigen exposure. Dr. Chang’s approach takes advantage that both the B lymphoblasts and the IgE-memory cells express high density of mIgE on the surface and thus are susceptible for Anti-CεmX targeting.
The team screened more than 4000 hybridoma clones to identify a few potential anti-CεmX candidates. Jiun-Bo performed a series of functional analyses, including, the binding to mIgE-expressing B cells, the ability to cause antibody-dependent cytolysis, the ability to induce apoptosis of mlgE-expressing cells, and others, to evaluate the applicability of the candidate Anti-CεmX monoclonal antibodies for future development. One of the best monoclonal antibodies, named “4B12”, was chosen for further characterization studies. The results were published in a paper in the journal of Immunology in 2010 (see paper: Unique Epitopes on CεmX in IgE–B Cell Receptors Are Potentially Applicable for Targeting IgE-Committed B Cells).
The antibody humanization task was performed by Wu Pheidias. The goal was to alter all of the constant regions and the framework segments of the variable regions of the mouse antibody 4B12 to corresponding structures of a human IgG1 antibody. The purpose is that to avoid a patient, after being administered 4B12, to make antibody response against the drug, which will consumes the drug and compromises the drug’s effectiveness. Most of the work was done at the level of the genes encoding 4B12. Pheidias was able to achieve this goal.
Prof. Chang expressed that he encourages his students and fellows to do a lot of independent thinking. He generally does not assign research projects and expects the students to evolve their own projects. His students discuss ideas among themselves and form research teams and distribute their individual responsibilities. For the present invention on anti-CεmX, the three students all have significant conceptual contributions and are legitimate co-inventors in the resulted patent application.
As it was publicly announced, a local biotech firm has licensed the Anti-CεmX technology pursuing commercial development. The story reported here should inspire many fellow researchers and students for pursuing research that have application potential. If all goes well and the anti-CεmX antibody is approved by regulatory agencies for sale, the team members can share part of the milestone payments and Academia Sinica will also make certain distribution of the royalty income.
However, the group is reserved in expressing such a prospect. They are well aware that new drug development is a long and formidable journey with major uncertainties on the way. Taking Xolair as an example, it took 16 years, going through many major obstacles, before being approved. The only sure thing is that the members will remain focused and devoted in their research pursuit.
Bravo! Prof. Chang and his team have provided a vivid example for many professors and researchers and their students in our commitment to participate in the national drive to develop biotech programs and industry in Taiwan.
(GRC web communications)