Sulfated Disaccharides Repair Toxic Protein Gain-of-Function, a New Approach to Treatment of Amyotrophic Lateral Sclerosis
- Details
- Published: Monday, 18 March 2024 10:00
In 2014, the charity event called the 'Ice Bucket Challenge' caused a sensation on social media, raising awareness about motor neuron diseases, especially Amyotrophic Lateral Sclerosis (ALS). Besides, Frontotemporal Dementia (FTD) is another type of dementia, second only to Alzheimer's disease worldwide. Both diseases are neurodegenerative diseases that primarily affect middle-aged people.
ALS patients experience progressive muscle atrophy and paralysis due to the degeneration of motor neurons in the spinal cord, brainstem, or motor cortex. Renowned scientist Stephen Hawking and former American baseball star Lou Gehrig are both ALS patients. FTD patients, on the other hand, exhibit symptoms such as behavioral deterioration and language impairments due to the degeneration of neurons in the frontal and temporal lobes. Hollywood action star Bruce Willis announced his retirement in 2022 due to this disease.
Although these two diseases differ in clinical symptoms, they share many pathological features and genetic variations. Clinical data shows that 95% of ALS and 70% of FTD patients are sporadic cases, making it difficult to analyze the underlying genetic variations related to pathogenesis. However, in familial cases, the most common genetic mutation for both diseases originates from hexanucleotide repeat expansion of the GGGGCC (G4C2) sequence in the non-coding region of the C9ORF72 gene.
Professor Yun-Ru (Ruby) Chen's laboratory at Genomics Research Center (GRC), Academia Sinica (AS) has long been dedicated to studying neurodegenerative diseases related to protein misfolding. Recently, her team published a story in the journal “Science Advances”, elucidating a new toxicity mechanism induced by C9ORF72 hexanucleotide repeat expansion translated poly-glycine-arginine (poly-GR) and poly-proline-arginine (poly-PR), successfully inhibiting their toxicity to neurons using sulfated disaccharides, and reducing neuronal degeneration.
Compared to the C9ORF72 gene in healthy individuals, the G4C2 repeats in ALS/FTD patients may expand from a few hundreds to even thousands of times. When G4C2 repeats are highly expanded, they can trigger a mechanism called "non-ATG-initiated translation" (RAN) leading to the production of five dipeptide repeats, including poly-GA, poly-GR, poly-GP, poly-PR, and poly-PA, which form inclusion bodies in cells after misfolding. Clinically, these proteinaceous inclusions have been found in various regions in the nervous system, including the hippocampus, frontal lobe, motor cortex, spinal cord, and cerebellum, with poly-GA being the most abundant one. The toxicity of poly-GA arises after accumulation, whereas the highly hydrophilic feature of poly-GR and poly-PR leads to toxicity mechanisms distinct from amyloid deposits, and even more toxic.
The first author of this paper, Ph.D. student Yu-Jen Chang, first synthesized different lengths of poly-GR/-PR using chemical synthesis in collaboration with the peptide synthesis facility at GRC, resembling different lengths of G4C2 repeats. Cell studies showed that when treating neuronal cells with poly-GR possessing repeats exceeding 25-30 times, the cell viability decreased, the plasma membrane and nuclear membrane were distrupted, vital mechanisms related to nucleic acids such as DNA replication, transcription, and protein translation, were interferred. These pathogenic mechanisms were further validated in neuroblastoma expressing poly-GR/-PR.
Collaborated with Dr. U-Ser Jeng's team at the National Synchrotron Radiation Research Center (NSRRC), the team further examined the structures of poly-GR and poly-PR in solution using TPS13A Small-Angle X-ray Scattering (SAXS) beamline. Molecular simulations and analysis of SAXS data were combined to propose characteristic models of the soft structures of poly-GR and poly-PR, systematically interpreting the relationship between generation of soft helical structures and composition and length of poly-GR and poly-PR. Structural analysis indicated that only poly-GR forms soft helical structures after reaching a specific length, which marks the beginning of its biological toxicity. This novel development of SAXS technique combined with molecular simulations represents a significant breakthrough in analyzing the structures of unstable protein molecules in solution.
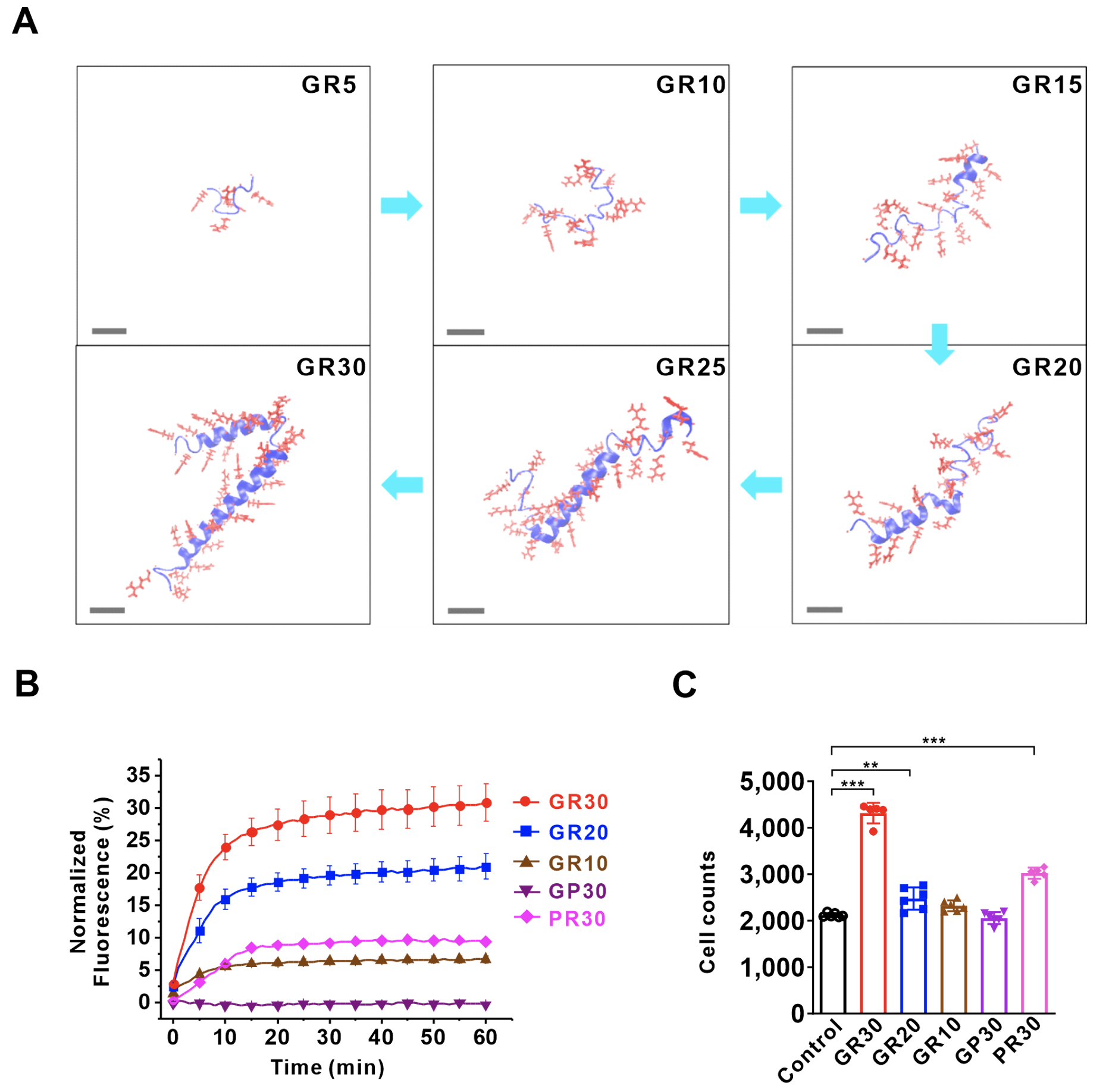 |
| Figure 1: Helical structure formed in long-chain poly-GR and its disruption to lipid bilayers. (A) When the repeat number of peptides exceeded 20, poly-GR clearly formed helical structure. Poly-GR and poly-PR caused damage to (B) artificial liposome and (C) cell membrane. |
Since mature neurons cannot proliferate, increasing cell viability is the goal of therapeutics. The research team utilized the library of heparan sulfate molecules established in the laboratory of Dr. Shang-Cheng Hung, Distinguished Research Fellow in GRC. From the screening, a sulfated disaccharide (sucrose octasulfate, SOS) showed the highest potency in reducing cytotoxicity. In collaboration with Dr. Hung-Chih Kuo from the Institute of Cellular and Organismic Biology (ICOB), AS, and Dr. Yi-Chung Lee from the Department of Neurology at Taipei Veterans General Hospital, the team successfully demonstrated the reversed neurotoxicity induced by poly-GR/-PR in motor neurons differentiated from ALS patients' pluripotent stem cells (C9ALS-iPS derived motor neurons).
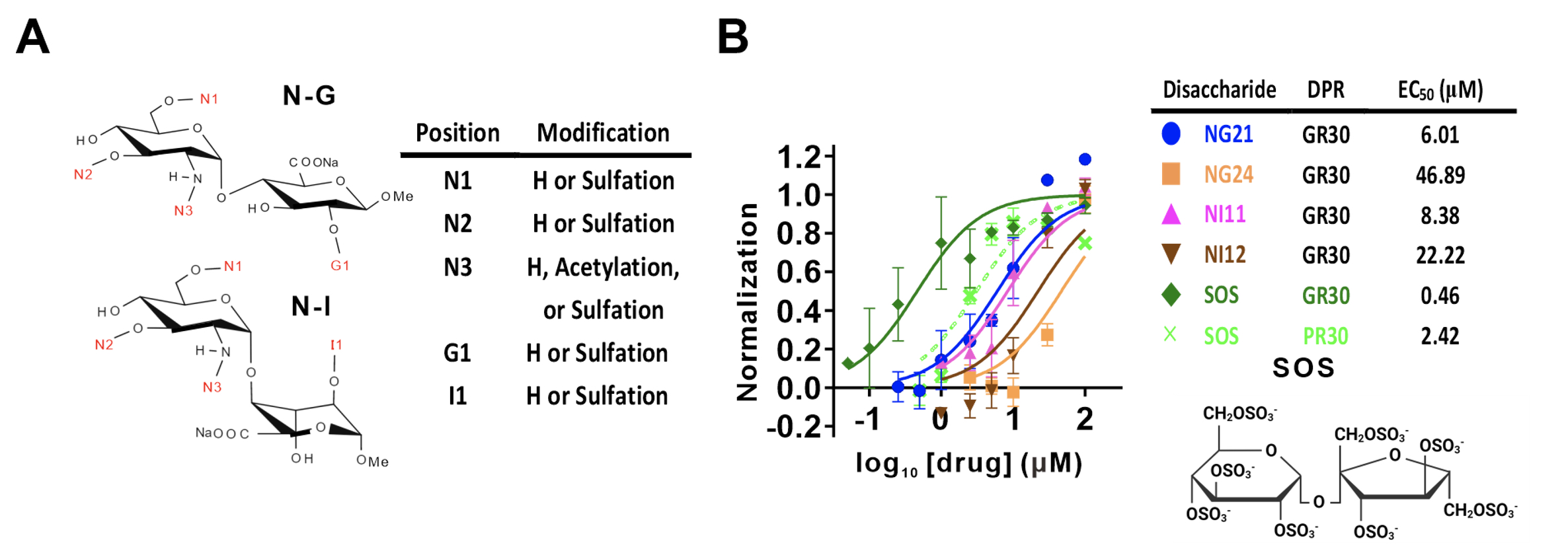 |
| Figure 2: The sulfated disaccharide selected from the heparan sulfate disaccharide library. (A) A schematic diagram of the heparan sulfate library. The positions and available modifications are shown. (B) Determination of the half-maximal effective concentration (EC50) and chemical structure of the most effective molecule, SOS, for reducing the cytotoxicity of poly-GR/-PR. |
Furthermore, the team collaborated with Dr. Chi-Kuang Yao from the Institute of Biological Chemistry (IBC), AS to conduct in vivo studies using transgenic Drosophila. After induction, the nervous system of Drosophila exhibited toxic poly-GR/-PR, leading to shortened lifespan and reduced locomotion. Subsequently, the sulfated disaccharide was treated to Drosophila and the results showed that Drosophila fed with the sulfated disaccharide lived 25% longer than those without. By observing the climbing ability of Drosophila, on the 13th day, 90% of the flies fed with the sulfated disaccharide were still active above the set threshold, while only half of those fed without the sulfated disaccharide remained active. Subsequent experiments on the motor cortex of wild-type mouse co-injected poly-GR and the compound also demonstrated positive results.
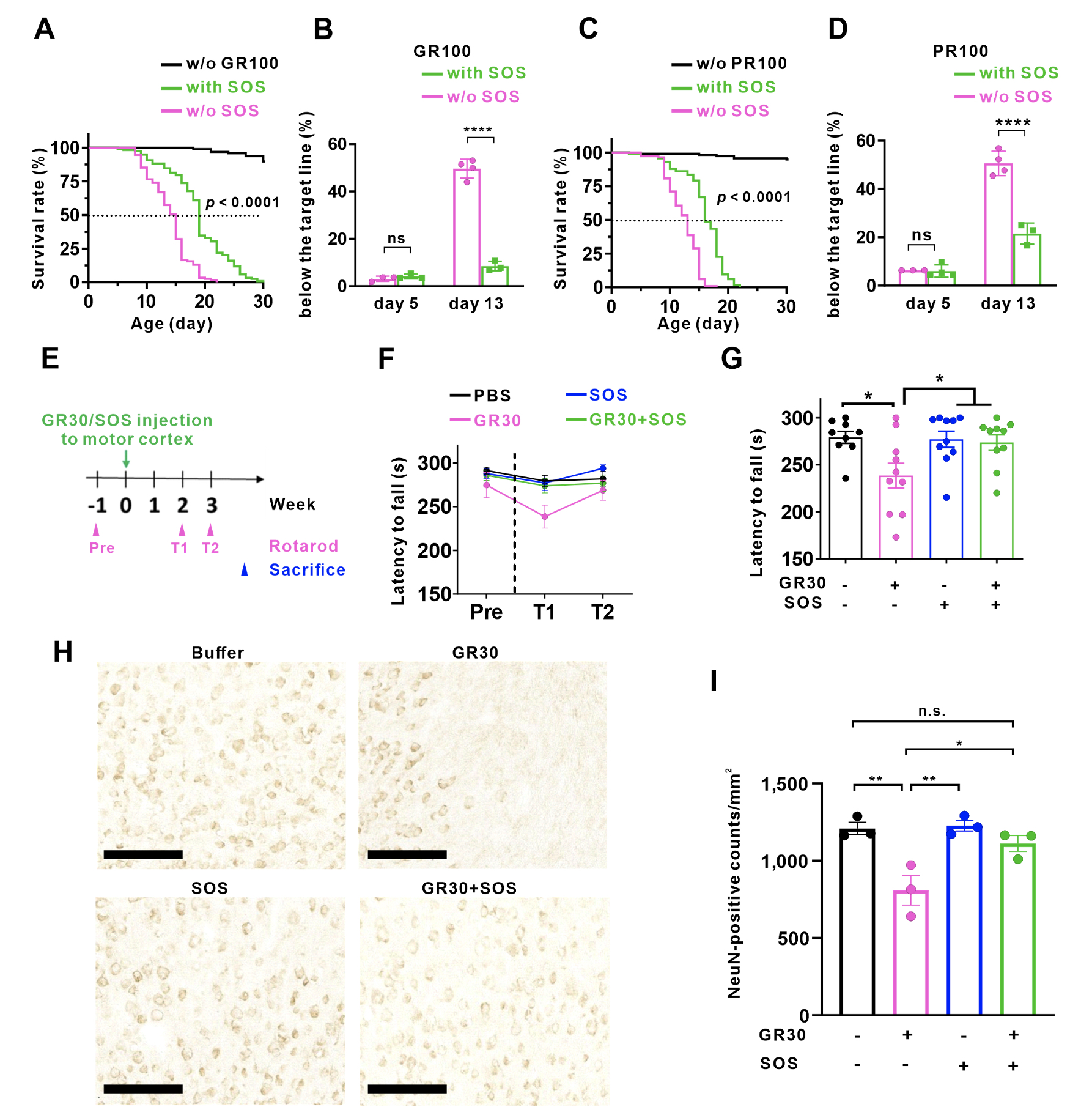 |
| Figure 3: Longevity and climbing assays of Drosophila and rotarod task and motor cortex staining of poly-GR/SOS injection mouse model. (A-D) Expression of poly-GR and poly-PR in the nervous system of Drosophila resulted in shortened lifespan and defective locomotion, which can be improved by addition of the sulfated disaccharide to their food. (E-I) Injection of poly-GR into the mouse motor cortex impaired the motor coordination and reduced the number of neurons, whereas, co-injection with the sulfated disaccharide prevents the damage caused by poly-GR. |
The cause of ALS is still not fully understood, with discovered genetic mutations accounting for only ~5% of cases, and ALS predominantly affects individuals aged 40 to 65. This study focuses on the C9ORF72 gene, which is the most common genetic mutation in familial cases of ALS, to investigate whether the toxicity mechanisms of poly-GR/-PR are related to the number of DNA repeats. The study provides insights into the minimal threshold of G4C2 repeat numbers in ALS patients and proposes the mechanism about the helical structure inducing damages to plasma membrane and nuclear membrane. Furthermore, it is advanced in discovering poly-GR/-PR interference with essential nucleotide mechanisms. Through experiments involving C9ALS-iPS derived motor neurons, Drosophila, and mice, the study provides the direction of sulfated disaccharides in ALS therapeutics. The research team aims to uncover more details of the pathogenic mechanisms to provide further potential therapeutics for neurodegenerative diseases.
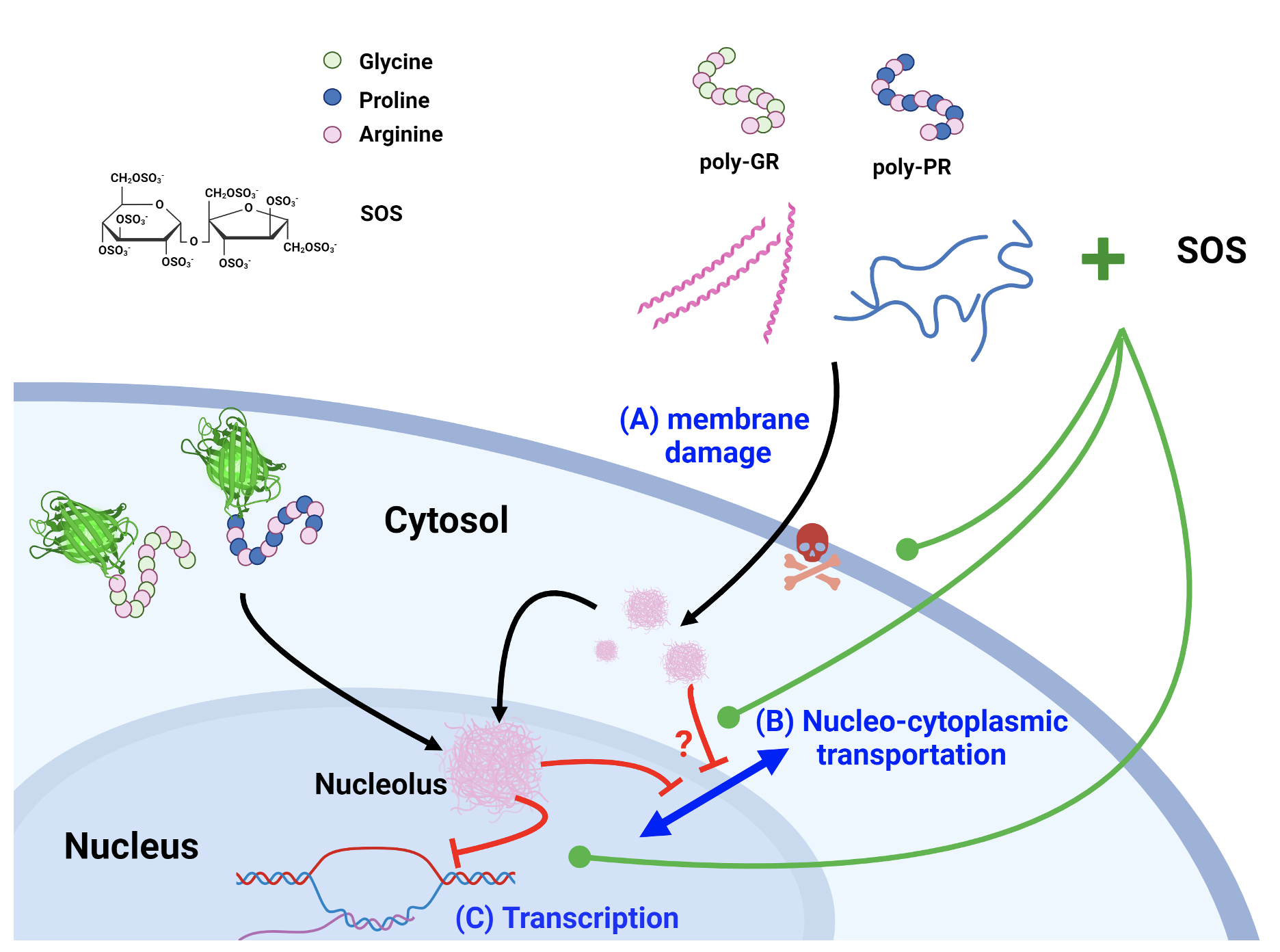 |
| Figure 4: Schematic representation of mechanisms involved in poly-GR/-PR toxicity and the potential therapeutic action of the sulfated disaccharide. Both poly-GR and poly-PR (A) disrupt cell membrane while the helical structure of long-chain poly-GR enhances this effect. Upon entry into the cell, they (B) inhibit nucleo-cytoplasmic transportation. Ultimately, poly-GR/-PR enter the nucleus, particularly in the nucleolus, where abundant poly-GR/-PR can be observed, thereby (C) inhibiting transcription. Upon addition of the sulfated disaccharide, these cytotoxic effects are neutralized by the binding to poly-GR/-PR, leading to increased cell viability. |
This research was led by Dr. Yun-Ru (Ruby) Chen from the GRC, AS with experiments primarily conducted by Ph.D. student Yu-Jen Chang from the Taiwan International Graduate Program (TIGP)- Interdisciplinary Neuroscience. Mouse experiments were carried out by research assistant Ms. Chi-Hua Yang, primary neurons were prepared by postdoctoral researcher Dr. Wei-Bin Lai, and pluripotent stem cells were differentiated by Dr. Ching-Yu Chuang. Collaborators include Dr. U-Ser Jeng from NSRRC, Dr. Shang-Cheng Hung at GRC, AS, Dr. Chi-Kuang Yao at IBC, AS, Dr. Hung-Chih Kuo at ICOB, AS, and Dr. Yi-Chung Lee from the Department of Neurology at Taipei Veterans General Hospital. A full study "Sulfated Disaccharide Protects Membrane and DNA Damages from Arginine-rich Dipeptide Repeats in ALS" was published in Science Advances and can be read online at: https://www.science.org/doi/10.1126/sciadv.adj0347