Pancreatic ductal adenocarcinoma (PDAC) is responsible for more than 90% of pancreatic cancer cases, just 11% of PDAC patients live for at least five years after their diagnosis. It is seldom detected at its early stages when it's most curable. Therefore, there is an urgent need for an in-depth understanding of the mechanisms involved in the progression of PDAC in order to develop new therapeutic strategy.
A research team led by Dr. Wen-Hwa Lee and Dr. Chun-Mei Hu in the Genomics Research Center show the first time that how PDAC tumor cells recruit nearby cells called fibroblasts to work as their helpers at tumor-stromal boundaries through direct tumor-fibroblast interaction. These interactions in PDAC progression enhance cancer cell migration and make the tumors more malignant. Blockage of direct contact between tumor cells and fibroblasts may represent a promising therapeutic strategy for patients with PDAC. This research will later be published in the journal "Nature Communications".
Tumor cells with diverse biological behaviors are influenced by stromal cells through secretory factors or direct cell-cell contact. PDAC is characterized by extensive desmoplasia with fibroblasts as the most essential components of the tumor microenvironment.
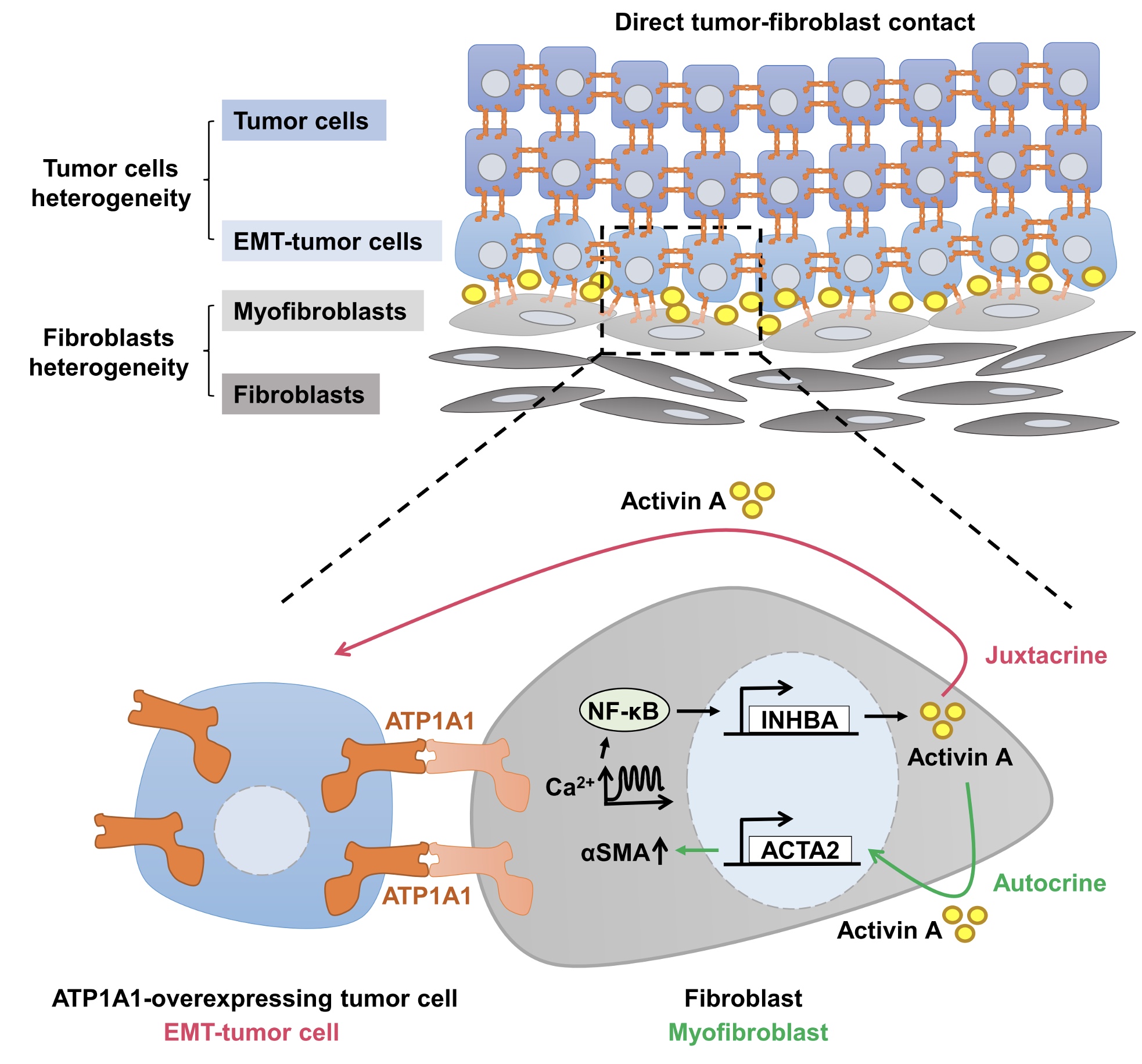 |
| Homophilic ATP1A1 interaction induces activin A to generate tumor cells with EMT plasticity and autocrine activin A for fibroblast self-activation, thereby facilitating collective invasion and metastasis of cancer cells |
“We observed enrichment of myofibroblasts in a juxta-tumoral position with tumor cell undergoing epithelial-mesenchymal transition (EMT) that facilitates invasion and correlates with a worse clinical prognosis in PDAC patients,” said Yi-Ing Chen, PhD, a postdoctoral researcher in the Dr. Hu’s Laboratory and first author of the study.
It was also noted that activin A is secreted from fibroblasts primarily upon direct contact with tumor cells. In general, activin A signaling promotes EMT, invasion, and metastatic growth of cancers.
“We assumed enrichment of activin A in tumor-stromal interface promotes EMT in tumor cells that facilitate invasion and metastasis. In addition, activin A gradient along tumor-stromal interface induce fibroblast activation.” said Dr. Chen.
To better understand which membrane protein was involved in contact-mediated activin A secretion, researchers examined membrane proteins found on several tumor cell lines were able to induce fibroblast activin A production. It is found out that ATP1A1 on tumor cell membranes is important for inducing activin A secretion from fibroblasts and EMT of tumor cells.
The results from this study revealed the initial step of pancreatic cancer metastasis. When ATP1A1 overexpressing tumor cells encounter surrounding stromal cells, they bind to ATP1A1 of fibroblasts and reorganize fibroblasts into a cancer-promoting mode. Dr. Chen explained, “ATP1A1 serves simultaneously as a signaling receptor and adhesion molecule during tumor-fibroblast contact.”
Homophilic ATP1A1 interaction induces activin A to generate tumor cells with EMT plasticity and autocrine activin A for fibroblast self-activation. These juxta-tumoral fibroblasts serve as a stromal niche to support EMT of tumor cells in the tumor invasion front and blood circulation, thereby facilitating collective invasion, treatment resistance, and metastasis of cancer cells, leading to poor clinical outcomes for patients.
Elucidating the mechanistic basis and subsequent effects of interaction between fibroblasts and PDAC tumor cells may provide a potential treatment approach for PDAC. Further analysis showed that silencing ATP1A1 expression or neutralizing activin A secretion suppressed tumor invasion and colonization. These findings pave the way for future therapeutic opportunities to inhibit metastasis by interfering with cell-cell interactions.
This research work was successfully completed by efforts from core team members including Drs. Wen-Hwa Lee, Chun-Mei Hu and Yi-Ing Chen. Appreciations to National Taiwan University physicians include: Drs. Yu-Wen Tien, Yu-Ting Chang, Ming-Chu Chang and Yung-Ming Jeng.
This study has been published in the Nature Communications. The paper titled: “Homophilic ATP1A1 Binding Induces Activin A Secretion to Promote EMT of Tumor Cells and Myo-fibroblast Activation” can be read online at: https://doi.org/10.1038/s41467-022-30638-4.